OHTrust Nano Ion Water by Nanoplus Limited (Cayman) Taiwan Branch Nanoplus 005-50
OHTrust Nano Ion Water by
Drug Labeling and Warnings
OHTrust Nano Ion Water by is a Otc medication manufactured, distributed, or labeled by Nanoplus Limited (Cayman) Taiwan Branch. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
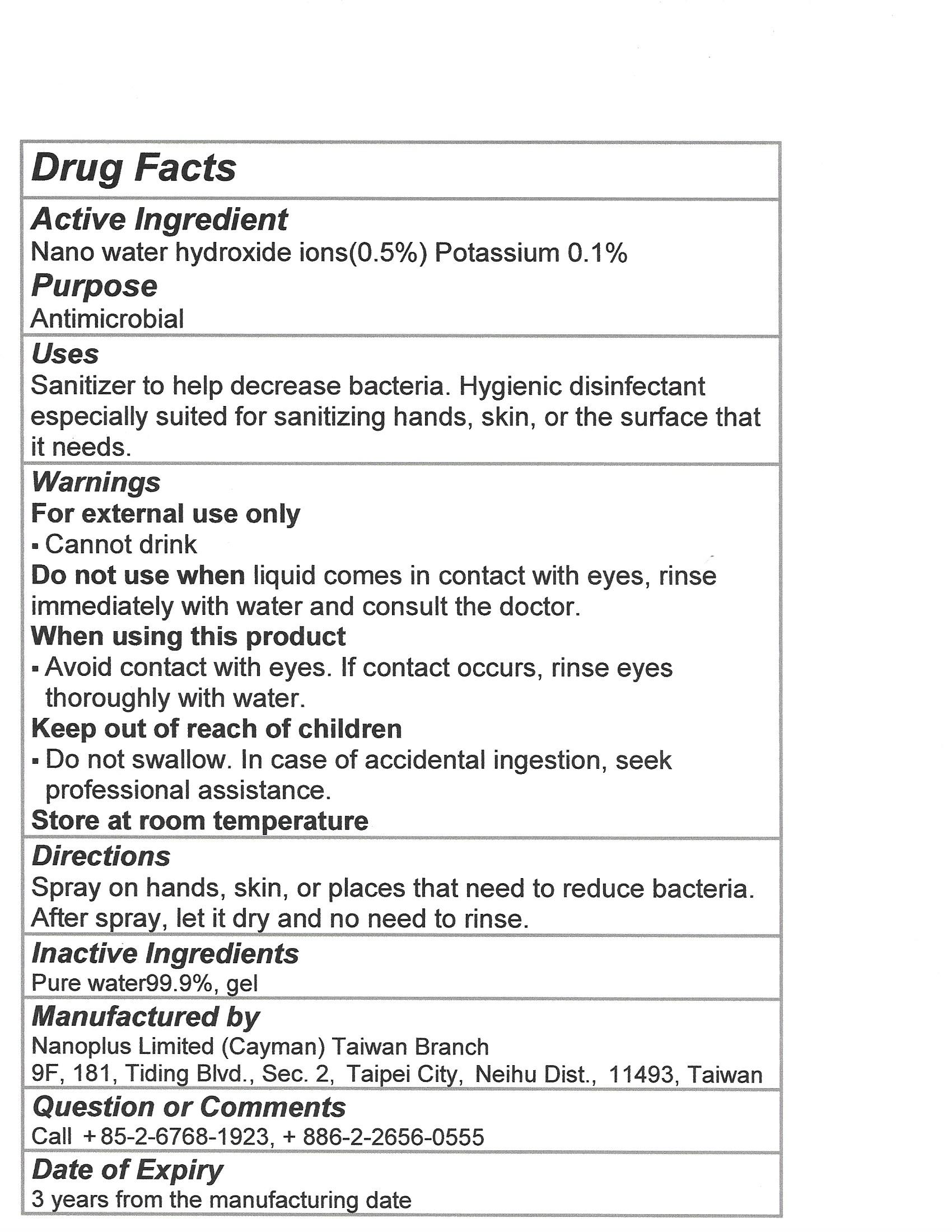
OHTRUST NANO ION WATER- hydroxide ions, potassium liquid
Nanoplus Limited (Cayman) Taiwan Branch
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Nanoplus 005-50
Use
Sanitizer to help decrease bacteria. Hygienic disinfectant especially suited for sanitizing hands, skin or the surface that it needs.
Do not use when liquid comes in contact with eyes, rinse immediately with water and consult the doctor
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
| OHTRUST NANO ION WATER
hydroxide ions, potassium liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Nanoplus Limited (Cayman) Taiwan Branch (656283417) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanoplus Limited (Cayman) Taiwan Branch | 656283417 | manufacture(70970-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 5000 mL NDC:
5000 mL NDC: