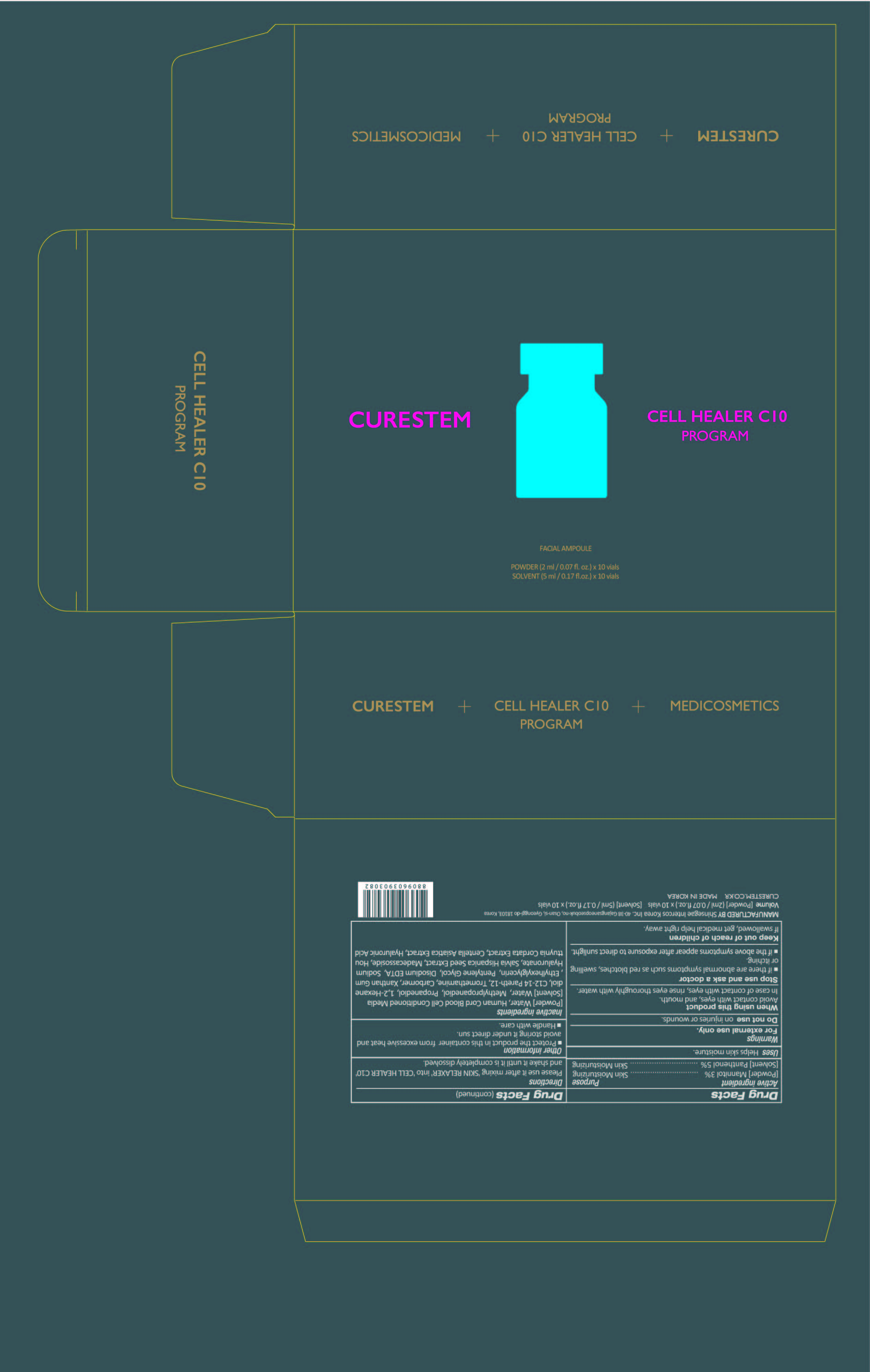
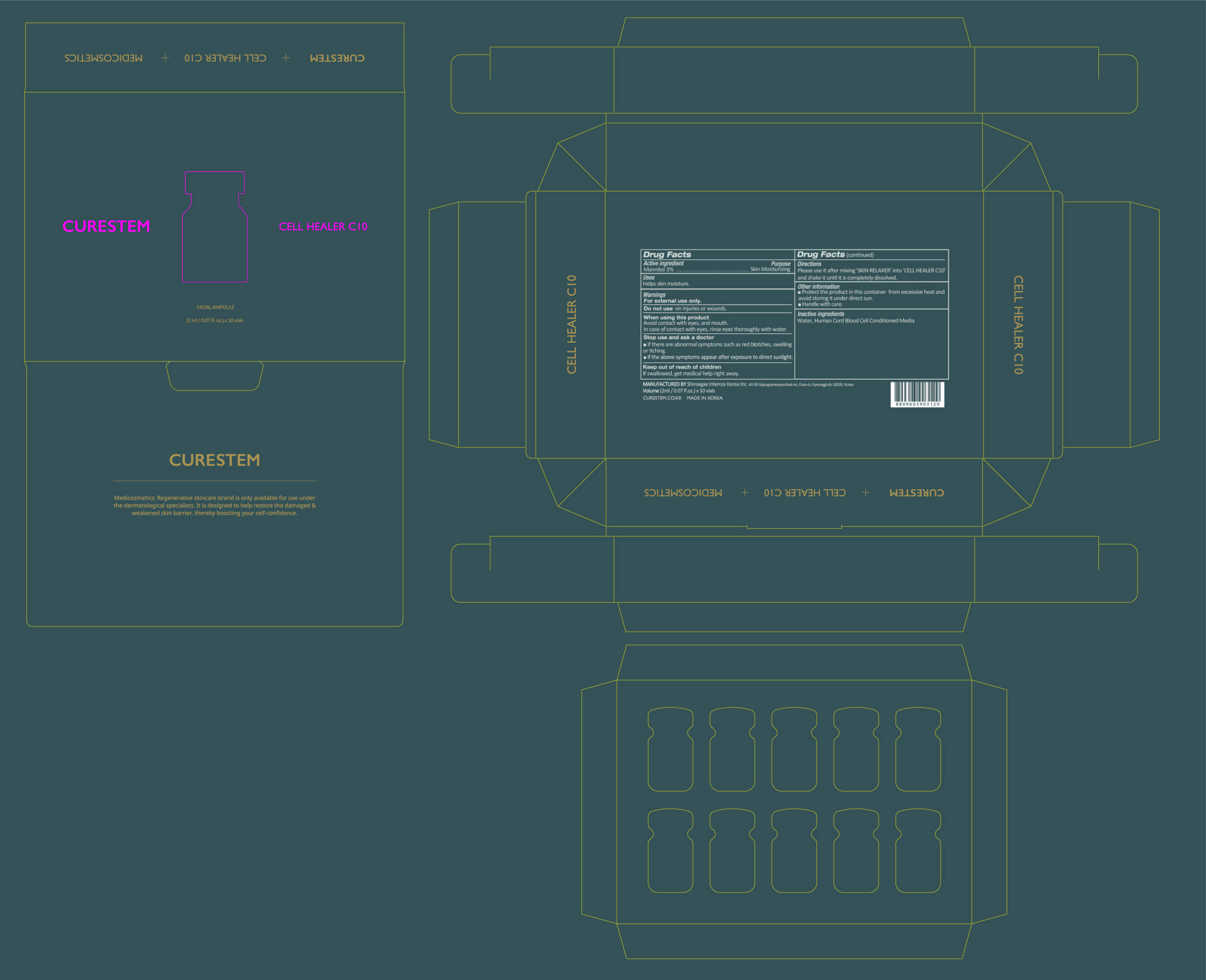
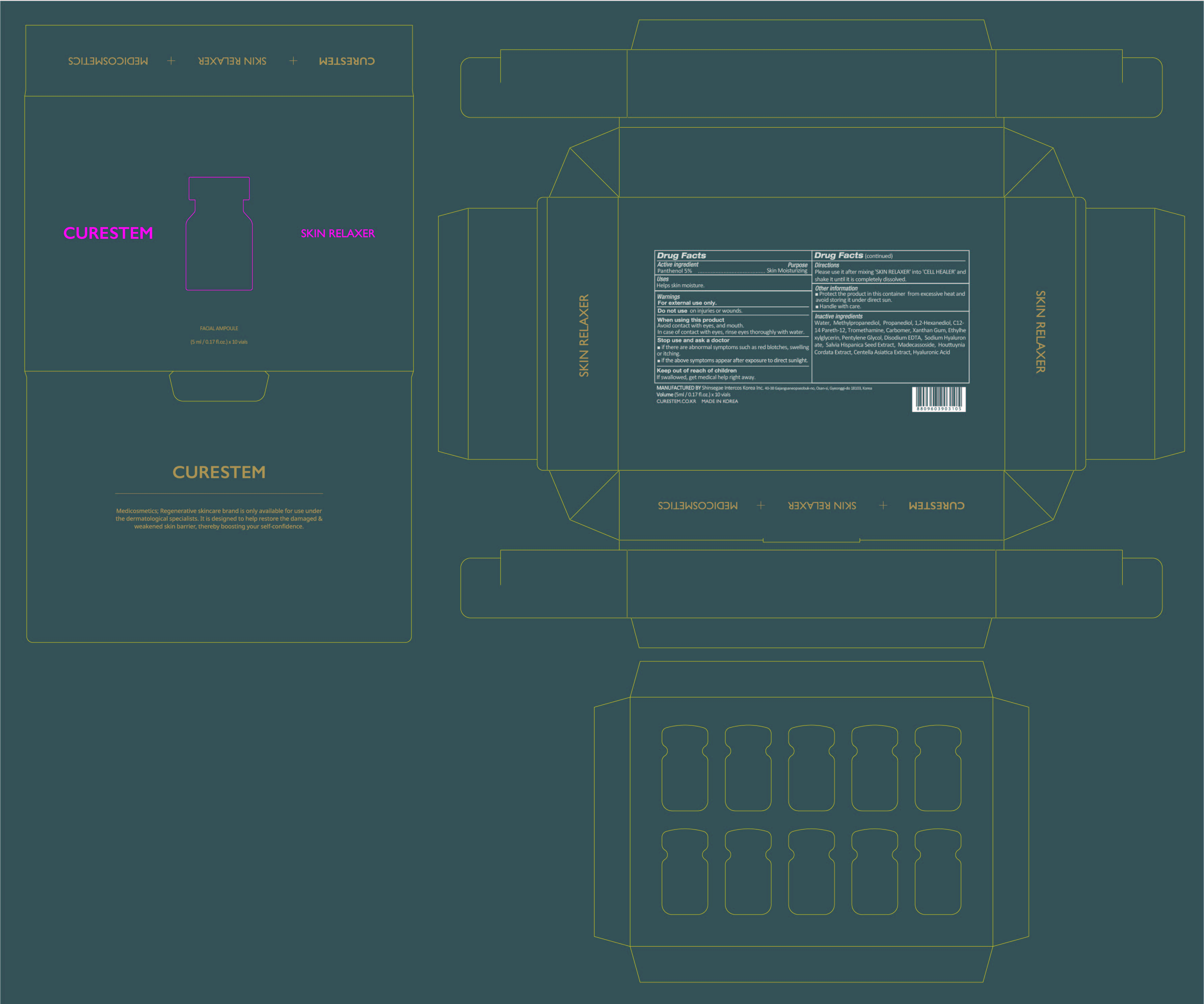
[De-listing 400-20] CURESTEM CELL HEALER C10 PROGRAM : 78521-400-20
CURESTEM CELL HEALER C10 PROGRAM by
Drug Labeling and Warnings
CURESTEM CELL HEALER C10 PROGRAM by is a Otc medication manufactured, distributed, or labeled by DNK CORPORATION LTD., Shinsegae Intercos Korea Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CURESTEM CELL HEALER C10 PROGRAM- mannitol, panthenol
DNK CORPORATION LTD.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
[De-listing 400-20] CURESTEM CELL HEALER C10 PROGRAM : 78521-400-20
When using this product
Avoid contact with eyes, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor
■ if there are abnormal symptoms such as red blotches, swelling or itching.
■ if the above symptoms appear after exposure to direct sunlight.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Please use it after mixing 'SKIN RELAXER' into 'CELL HEALER C10'
and shake it until it is completely dissolved.
Other information
■ Protect the product in this container from excessive heat and
avoid storing it under direct sun.
■ Handle with care.
Inactive ingredients
[Powder] Water, Human Cord Blood Cell Conditioned Media
[Solvent] Water, Methylpropanediol, Propanediol, 1,2-Hexanediol,
C12-14 Pareth-12, Tromethamine, Carbomer, Xanthan Gum,
Ethylhexylglycerin, Pentylene Glycol, Disodium EDTA, Sodium Hyaluronate,
Salvia Hispanica Seed Extract, Madecassoside, Houttuynia Cordata Extract,
Centella Asiatica Extract, Hyaluronic Acid
| CURESTEM CELL HEALER C10 PROGRAM
mannitol, panthenol kit |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - DNK CORPORATION LTD. (695538778) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shinsegae Intercos Korea Inc. | 694526100 | manufacture(78521-200, 78521-300, 78521-400) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.