MEDLINE- cetirizine hcl tablet
Medline by
Drug Labeling and Warnings
Medline by is a Otc medication manufactured, distributed, or labeled by Medline Industries, LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
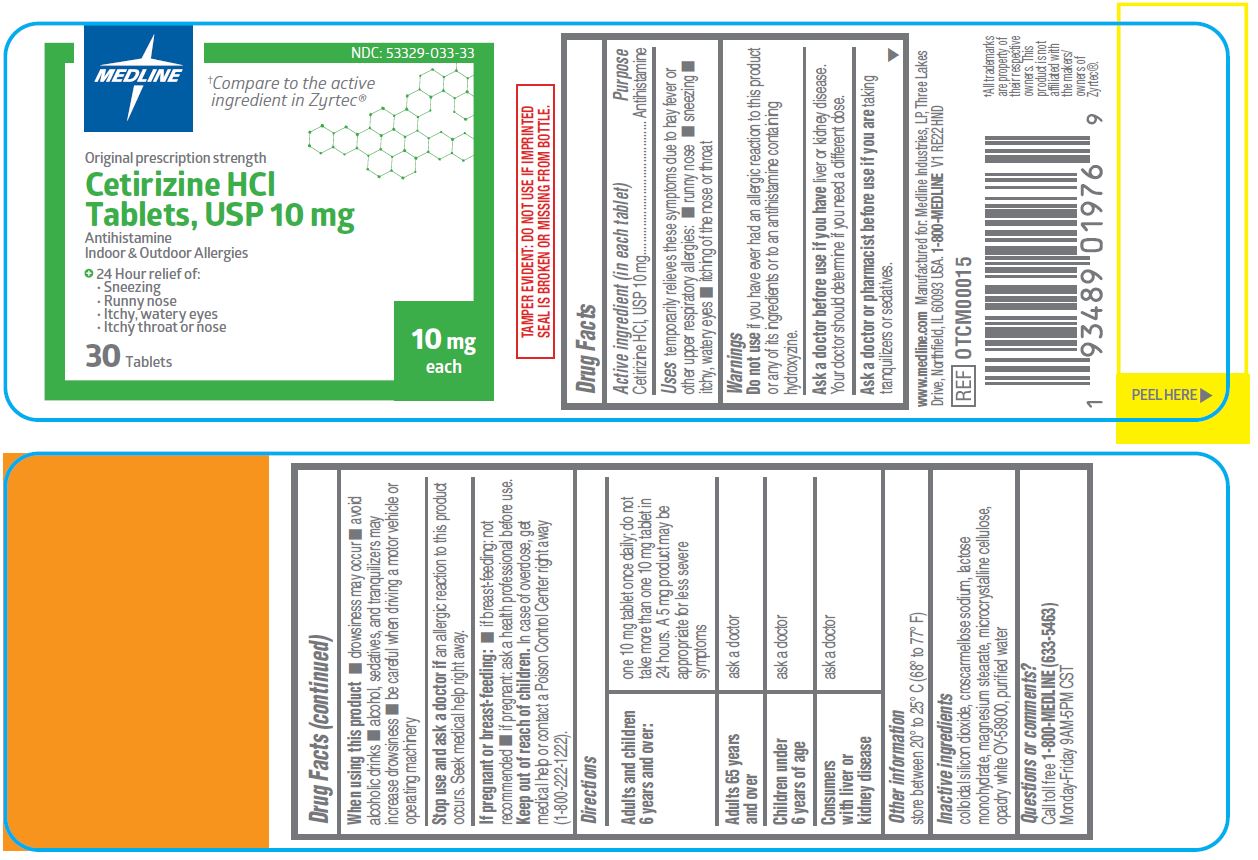
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- Manufacturing Information
- Package Label
-
INGREDIENTS AND APPEARANCE
MEDLINE
cetirizine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53329-033 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white ((to off white)) Score no score Shape RECTANGLE ((rounded off rectangular shaped)) Size 9mm Flavor Imprint Code G;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53329-033-33 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2020 2 NDC: 53329-033-38 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2020 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209274 05/01/2020 Labeler - Medline Industries, LP (025460908)
Trademark Results [Medline]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEDLINE 86846058 5021107 Live/Registered |
IPEG, INC. 2015-12-11 |
 MEDLINE 86488830 5166882 Live/Registered |
Medline Industries, Inc. 2014-12-23 |
 MEDLINE 86488829 5171995 Live/Registered |
Medline Industries, Inc. 2014-12-23 |
 MEDLINE 86382367 not registered Dead/Abandoned |
IPEG, INC. 2014-09-02 |
 MEDLINE 79308544 not registered Live/Pending |
Singulus Technologies AG 2020-12-28 |
 MEDLINE 78790084 3301915 Live/Registered |
Medline Industries, Inc. 2006-01-12 |
 MEDLINE 78790077 3365696 Live/Registered |
Medline Industries, Inc. 2006-01-12 |
 MEDLINE 78790069 3343906 Live/Registered |
Medline Industries, Inc. 2006-01-12 |
 MEDLINE 78789639 3311898 Live/Registered |
Medline Industries, Inc. 2006-01-12 |
 MEDLINE 78476716 3311664 Live/Registered |
MEDLINE INDUSTRIES, INC. 2004-08-31 |
 MEDLINE 77978091 3710539 Live/Registered |
Medline Industries, Inc. 2008-09-03 |
 MEDLINE 77561183 3778944 Live/Registered |
Medline Industries, Inc. 2008-09-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.