BRONKAID DUAL ACTION FORMULA- ephedrine sulfate and guaifenesin tablet, coated
Bronkaid by
Drug Labeling and Warnings
Bronkaid by is a Otc medication manufactured, distributed, or labeled by Foundation Consumer Healthcare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Asthma alert
Because asthma may be life threatening, see a doctor if you:
- are not better in 60 minutes
- get worse
- need more than 6 caplets in 24 hours
- use more than 4 caplets in 24 hours for more than 3 days a week
- have more than 2 asthma attacks in a week. These may be signs that your asthma is getting worse.
These may be signs that your asthma is getting worse.
- This product will not give you asthma relief as quickly as an inhaled bronchodilator.
Do not use
- unless a doctor said you have asthma
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- ever been hospitalized for asthma
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- seizures
- narrow angle glaucoma
- a psychiatric or emotional condition
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- a cough which is accompanied by excessive phlegm (mucus)
Ask a doctor or pharmacist before use if you are
- taking prescription drugs for asthma, obesity, weight control, depression, or psychiatric or emotional conditions
- taking any drug that contains phenylephrine, pseudoephedrine, ephedrine, or caffeine (such as for allergy, cough-cold, or pain)
When using this product
- your blood pressure or heart rate may go up. This could increase your risk of heart attack or stroke, which may cause death.
-
your risk of heart attack or stroke increases if you:
- have a history of high blood pressure or heart disease
- take this product more frequently or take more than the recommended dose
- avoid foods or beverages that contain caffeine
- avoid dietary supplements containing ingredients reported or claimed to have stimulant effect
Stop use and ask a doctor if
- your asthma is getting worse (see Asthma Alert)
- you have difficulty sleeping
- you have a rapid heart beat
- you have tremors, nervousness, or seizure
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 24 Caplet Blister Pack Carton
NDC: 69536-425-24
Ephedrine Sulfate/Bronchodilator
Guaifenesin/ExpectorantBRONKAID®
STRONGEST FORMULA
available in a 1 caplet doseDUAL ACTION FORMULA:
- ✓ Restores Free Breathing
- ✓ Loosens Phlegm
Relief of Mild
Intermittent
Asthma SymptomsPHARMACIST
RECOMMENDED24
CAPLETS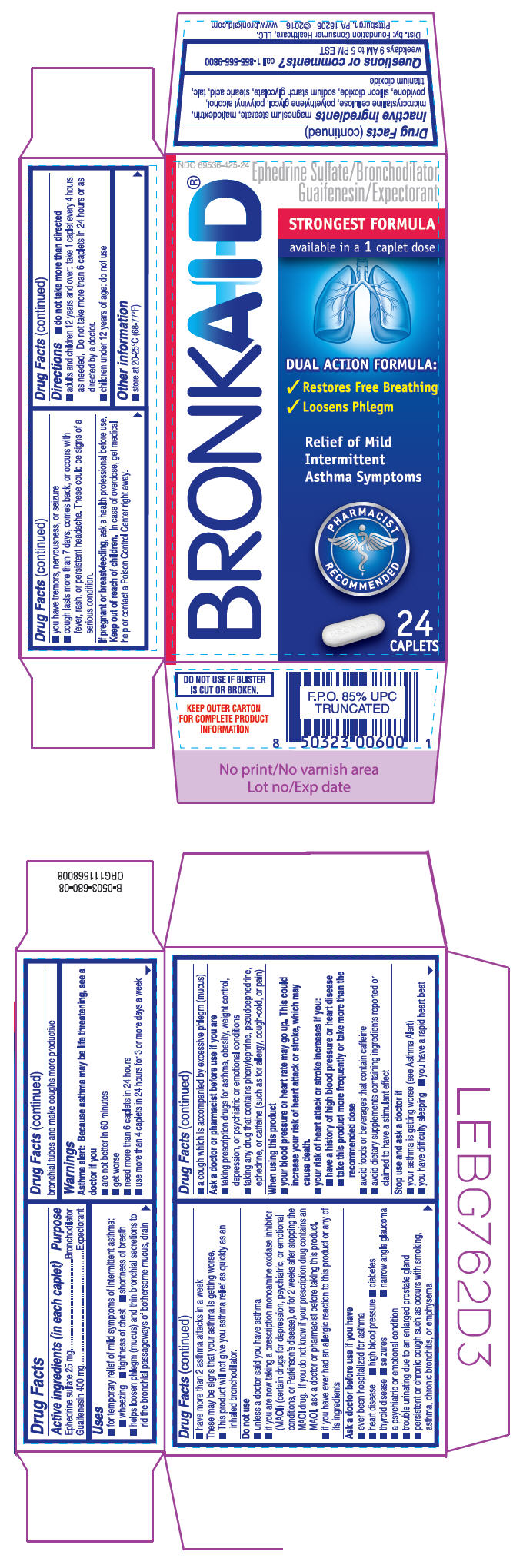
-
INGREDIENTS AND APPEARANCE
BRONKAID DUAL ACTION FORMULA
ephedrine sulfate and guaifenesin tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69536-425 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPHEDRINE SULFATE (UNII: U6X61U5ZEG) (EPHEDRINE - UNII:GN83C131XS) EPHEDRINE SULFATE 25 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg Inactive Ingredients Ingredient Name Strength MALTODEXTRIN (UNII: 7CVR7L4A2D) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape OVAL Size 18mm Flavor Imprint Code BRONKAID Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69536-425-24 4 in 1 CARTON 01/01/2006 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 69536-425-60 5 in 1 CARTON 01/01/2006 2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 01/01/2006 Labeler - Foundation Consumer Healthcare LLC (079675882)
Trademark Results [Bronkaid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRONKAID 72202920 0788673 Live/Registered |
DREW PHARMACAL CO., INC. 1964-09-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.