UPTRAVI- selexipag tablet, coated UPTRAVI TITRATION PACK- selexipag kit
UPTRAVI by
Drug Labeling and Warnings
UPTRAVI by is a Prescription medication manufactured, distributed, or labeled by Actelion Pharmaceuticals US, Inc., Excella GmbH, Almac Sciences Limited, Butterworth Laboratories Ltd, Almac Pharma Services Limited, SGS Institut Fresenius GmbH, EUROFINS AMATSIGROUP SAS, Wickham Micro Limited, Eurofins Amatsi Analytics, Confarma France, Konapharma AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use UPTRAVI® safely and effectively. See full prescribing information for UPTRAVI®.
UPTRAVI® (selexipag) tablets, for oral use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
INDICATIONS AND USAGE
UPTRAVI® is a prostacyclin receptor agonist indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to delay disease progression and reduce the risk of hospitalization for PAH. (1.1)
DOSAGE AND ADMINISTRATION
- Starting dose: 200 mcg twice daily. (2.1)
- Increase the dose by 200 mcg twice daily at weekly intervals to the highest tolerated dose up to 1600 mcg twice daily. (2.1)
- Maintenance dose is determined by tolerability. (2.1)
- Moderate hepatic impairment: Starting dose 200 mcg once daily, increase the dose by 200 mcg once daily at weekly intervals to the highest tolerated dose up to 1600 mcg. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 200 mcg, 400 mcg, 600 mcg, 800 mcg, 1000 mcg, 1200 mcg, 1400 mcg, 1600 mcg. (3)
WARNINGS AND PRECAUTIONS
Pulmonary edema in patients with pulmonary veno-occlusive disease. If confirmed, discontinue treatment. (5.1)
ADVERSE REACTIONS
Adverse reactions occurring more frequently (≥5%) on UPTRAVI compared to placebo are headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in extremity, and flushing. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Actelion at 1-866-228-3546 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Moderate CYP2C8 inhibitors (e.g., clopidogrel, deferasirox and teriflunomide) increase exposure to the active metabolite of UPTRAVI. Reduce the dosing of UPTRAVI to once daily (2.4,7.1,12.3).
- CYP2C8 inducers (e.g., rifampin) decrease exposure to the active metabolite. Increase up to twice the dose of UPTRAVI (7.2, 12.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Interruptions and Discontinuations
2.3 Dosage Adjustment in Patients with Hepatic Impairment
2.4 Dosage Adjustment with Co-administration of Moderate CYP2C8 Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pulmonary Veno-Occlusive Disease (PVOD)
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP2C8 Inhibitors
7.2 CYP2C8 Inducers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
8.7 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
UPTRAVI is indicated for the treatment of pulmonary arterial hypertension (PAH, WHO Group I) to delay disease progression and reduce the risk of hospitalization for PAH.
Effectiveness was established in a long-term study in PAH patients with WHO Functional Class II-III symptoms.
Patients had idiopathic and heritable PAH (58%), PAH associated with connective tissue disease (29%), PAH associated with congenital heart disease with repaired shunts (10%) [see Clinical Studies (14.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended starting dose of UPTRAVI is 200 micrograms (mcg) given twice daily. Tolerability may be improved when taken with food [see Clinical Pharmacology (12.3)].
Increase the dose in increments of 200 mcg twice daily, usually at weekly intervals, to the highest tolerated dose up to 1600 mcg twice daily. If a patient reaches a dose that cannot be tolerated, the dose should be reduced to the previous tolerated dose.
Do not split, crush, or chew tablets.
2.2 Interruptions and Discontinuations
If a dose of medication is missed, patients should take a missed dose as soon as possible unless the next dose is within the next 6 hours.
If treatment is missed for 3 days or more, restart UPTRAVI at a lower dose and then retitrate.
2.3 Dosage Adjustment in Patients with Hepatic Impairment
No dose adjustment of UPTRAVI is necessary for patients with mild hepatic impairment (Child-Pugh class A).
For patients with moderate hepatic impairment (Child-Pugh class B), the starting dose of UPTRAVI is 200 mcg once daily. Increase in increments of 200 mcg once daily at weekly intervals, as tolerated [see Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
Avoid use of UPTRAVI in patients with severe hepatic impairment (Child-Pugh class C).
2.4 Dosage Adjustment with Co-administration of Moderate CYP2C8 Inhibitors
When co-administered with moderate CYP2C8 inhibitors (e.g., clopidogrel, deferasirox and teriflunomide), reduce the dosing of UPTRAVI to once daily. Revert back to twice daily dosing frequency of UPTRAVI when co-administration of moderate CYP2C8 inhibitor is stopped [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
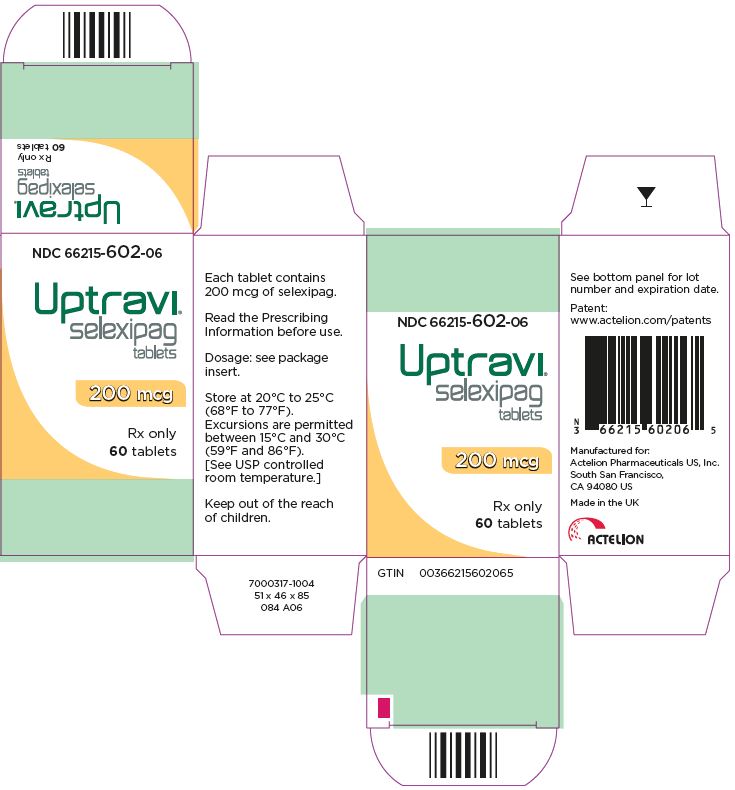
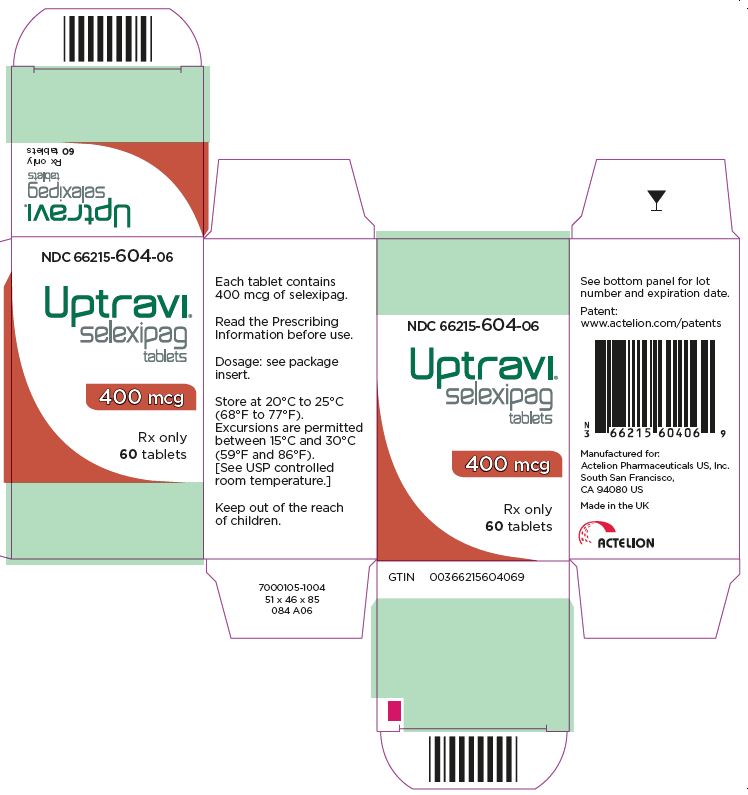
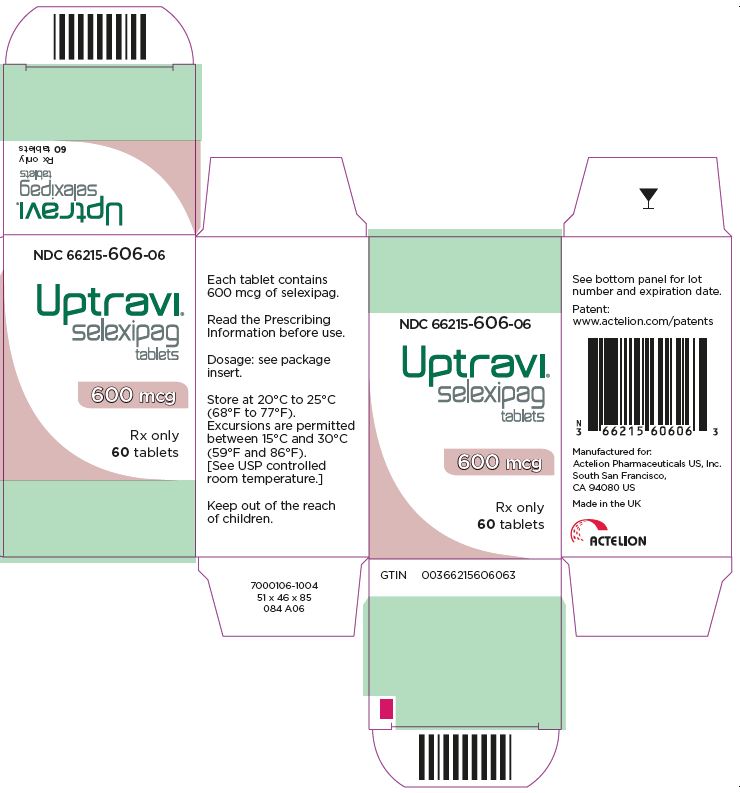
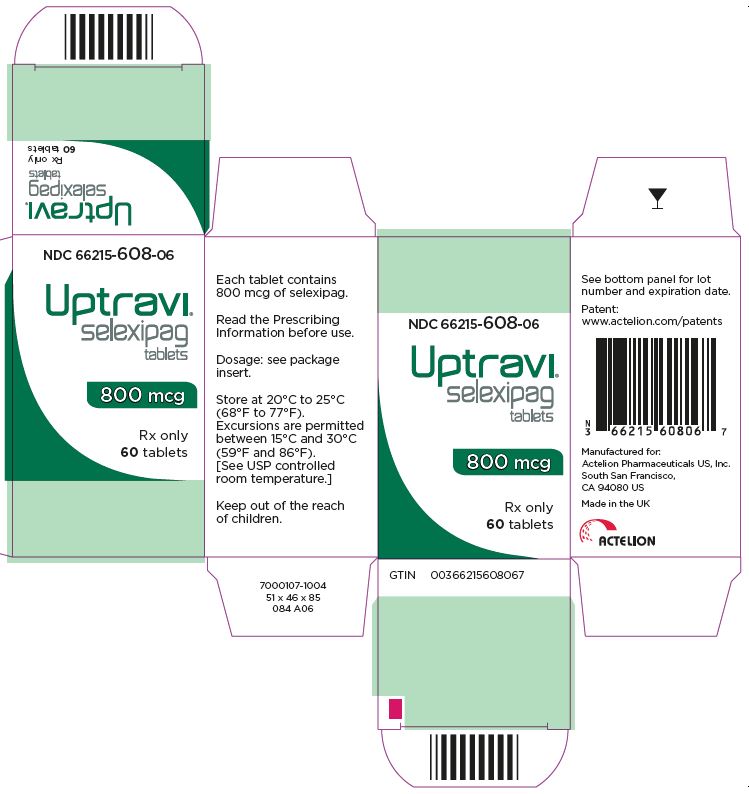
UPTRAVI is available in the following strengths:
- – 200 mcg [Light yellow tablet debossed with 2]
- – 400 mcg [Red tablet debossed with 4]
- – 600 mcg [Light violet tablet debossed with 6]
- – 800 mcg [Green tablet debossed with 8]
- – 1000 mcg [Orange tablet debossed with 10]
- – 1200 mcg [Dark violet tablet debossed with 12]
- – 1400 mcg [Dark yellow tablet debossed with 14]
- – 1600 mcg [Brown tablet debossed with 16]
-
4 CONTRAINDICATIONS
Concomitant use of strong inhibitors of CYP2C8 (e.g., gemfibrozil) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of UPTRAVI has been evaluated in a long-term, placebo-controlled study enrolling 1156 patients with symptomatic PAH (GRIPHON study) [see Clinical Studies (14)]. The exposure to UPTRAVI in this trial was up to 4.2 years with median duration of exposure of 1.4 years.
Table 1 presents adverse reactions more frequent on UPTRAVI than on placebo by ≥3%.
Table 1 Adverse Reactions UPTRAVI Placebo Adverse Reaction N=575 N=577 Headache 65% 32% Diarrhea 42% 18% Jaw pain 26% 6% Nausea 33% 18% Myalgia 16% 6% Vomiting 18% 9% Pain in extremity 17% 8% Flushing 12% 5% Arthralgia 11% 8% Anemia 8% 5% Decreased appetite 6% 3% Rash 11% 8% These adverse reactions are more frequent during the dose titration phase.
Hyperthyroidism was observed in 1% (n=8) of patients on UPTRAVI and in none of the patients on placebo.
Laboratory Test Abnormalities
Hemoglobin
In a Phase 3 placebo-controlled study in patients with PAH, mean absolute changes in hemoglobin at regular visits compared to baseline ranged from –0.34 to –0.02 g/dL in the selexipag group compared to –0.05 to 0.25 g/dL in the placebo group. A decrease in hemoglobin concentration to below 10 g/dL was reported in 8.6% of patients treated with selexipag and 5.0% of placebo-treated patients.
Thyroid function tests
In a Phase 3 placebo-controlled study in patients with PAH, a reduction (up to –0.3 MU/L from a baseline median of 2.5 MU/L) in median thyroid-stimulating hormone (TSH) was observed at most visits in the selexipag group. In the placebo group, little change in median values was apparent. There were no mean changes in triiodothyronine or thyroxine in either group.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of UPTRAVI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Symptomatic hypotension
-
7 DRUG INTERACTIONS
7.1 CYP2C8 Inhibitors
Concomitant administration with gemfibrozil, a strong inhibitor of CYP2C8, doubled exposure to selexipag and increased exposure to the active metabolite by approximately 11-fold. Concomitant administration of UPTRAVI with strong inhibitors of CYP2C8 (e.g., gemfibrozil) is contraindicated [see Contraindications (4) and Clinical Pharmacology (12.3)].
Concomitant administration of UPTRAVI with clopidogrel, a moderate inhibitor of CYP2C8, had no relevant effect on the exposure to selexipag and increased the exposure to the active metabolite by approximately 2.7-fold [see Clinical Pharmacology (12.3)]. Reduce the dosing of UPTRAVI to once daily in patients on a moderate CYP2C8 inhibitor [see Dosage and Administration (2.4)].
7.2 CYP2C8 Inducers
Concomitant administration with an inducer of CYP2C8 and UGT 1A3 and 2B7 enzymes (rifampin) halved exposure to the active metabolite. Increase dose up to twice of UPTRAVI when co-administered with rifampin. Reduce UPTRAVI when rifampin is stopped [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with UPTRAVI in pregnant women. Animal reproduction studies performed with selexipag showed no clinically relevant effects on embryofetal development and survival. A slight reduction in maternal as well as in fetal body weight was observed when pregnant rats were administered selexipag during organogenesis at a dose producing an exposure approximately 47 times that in humans at the maximum recommended human dose. No adverse developmental outcomes were observed with oral administration of selexipag to pregnant rabbits during organogenesis at exposures up to 50 times the human exposure at the maximum recommended human dose.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Pregnant rats were treated with selexipag using oral doses of 2, 6, and 20 mg/kg/day (up to 47 times the exposure at the maximum recommended human dose of 1600 mcg twice daily on an area under the curve [AUC] basis) during the period of organogenesis (gestation days 7 to 17). Selexipag did not cause adverse developmental effects to the fetus in this study. A slight reduction in fetal body weight was observed in parallel with a slight reduction in maternal body weight at the high dose.
Pregnant rabbits were treated with selexipag using oral doses of 3, 10, and 30 mg/kg (up to 50 times the exposure to the active metabolite at the maximum recommended human dose of 1600 mcg twice daily on an AUC basis) during the period of organogenesis (gestation days 6 to 18). Selexipag did not cause adverse developmental effects to the fetus in this study.
8.2 Lactation
It is not known if UPTRAVI is present in human milk. Selexipag or its metabolites were present in the milk of rats. Because many drugs are present in the human milk and because of the potential for serious adverse reactions in nursing infants, discontinue nursing or discontinue UPTRAVI.
8.5 Geriatric Use
Of the 1368 subjects in clinical studies of UPTRAVI 248 subjects were 65 years of age and older, while 19 were 75 and older. No overall differences were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity cannot be ruled out.
8.6 Patients with Hepatic Impairment
No adjustment to the dosing regimen is needed in patients with mild hepatic impairment (Child-Pugh class A).
A once-daily regimen is recommended in patients with moderate hepatic impairment (Child-Pugh class B) due to the increased exposure to selexipag and its active metabolite. There is no experience with UPTRAVI in patients with severe hepatic impairment (Child-Pugh class C). Avoid use of UPTRAVI in patients with severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.7 Patients with Renal Impairment
No adjustment to the dosing regimen is needed in patients with estimated glomerular filtration rate > 15 mL/min/1.73 m2.
There is no clinical experience with UPTRAVI in patients undergoing dialysis or in patients with glomerular filtration rates < 15 mL/min/1.73 m2 [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
UPTRAVI (selexipag) is a selective non-prostanoid IP prostacyclin receptor agonist. The chemical name of selexipag is 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}-N-(methylsulfonyl) acetamide. It has a molecular formula of C26H32N4O4S and a molecular weight of 496.62. Selexipag has the following structural formula:
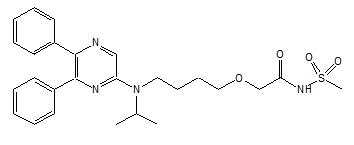
Selexipag is a pale yellow crystalline powder that is practically insoluble in water. In the solid state selexipag is very stable, is not hygroscopic, and is not light sensitive.
Depending on the dose strength, each round film-coated tablet for oral administration contains 200, 400, 600, 800, 1000, 1200, 1400, or 1600 mcg of selexipag. The tablets include the following inactive ingredients: D-mannitol, corn starch, low substituted hydroxypropylcellulose, hydroxypropylcellulose, and magnesium stearate. The tablets are film coated with a coating material containing hypromellose, propylene glycol, titanium dioxide, carnauba wax along with mixtures of iron oxide red, iron oxide yellow or iron oxide black.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Selexipag is an oral prostacyclin receptor (IP receptor) agonist that is structurally distinct from prostacyclin. Selexipag is hydrolyzed by carboxylesterase 1 to yield its active metabolite, which is approximately 37-fold as potent as selexipag. Selexipag and the active metabolite are selective for the IP receptor versus other prostanoid receptors (EP1-4, DP, FP, and TP).
12.2 Pharmacodynamics
Cardiac electrophysiology:
At the maximum tolerated dose of 1600 mcg twice daily, selexipag does not prolong the QT interval to any clinically relevant extent.
Platelet aggregation:
Both selexipag and its active metabolite caused concentration-dependent inhibition of platelet aggregation in vitro with an IC50 of 5.5 µM and 0.21 µM, respectively. However, at clinically relevant concentrations, there was no effect on platelet aggregation test parameters as seen following multiple-dose administrations of selexipag in healthy subjects from 400 mcg up to 1800 mcg twice daily.
Pulmonary hemodynamics:
A Phase 2 clinical study assessed hemodynamic variables after 17 weeks of treatment in patients with PAH WHO Functional Class II–III and concomitantly receiving endothelin receptor antagonists (ERAs) and/or phosphodiesterase type 5 (PDE-5) inhibitors. Patients titrating selexipag to an individually tolerated dose (200 mcg twice daily increments up to 800 mcg twice daily) (N=33) achieved a statistically-significant mean reduction in pulmonary vascular resistance of 30.3% (95% confidence interval [CI] –44.7%, –12.2%) and an increase in cardiac index (median treatment effect) of 0.41 L/min/m2 (95% CI 0.10, 0.71) compared to placebo (N=10).
12.3 Pharmacokinetics
The pharmacokinetics of selexipag and its active metabolite have been studied primarily in healthy subjects. The pharmacokinetics of selexipag and the active metabolite, after both single- and multiple-dose administration, were dose-proportional up to a single dose of 800 mcg and multiple doses of up to 1800 mcg twice daily.
In healthy subjects, inter-subject variability in exposure (area under the curve over a dosing interval, AUC) at steady-state was 43% and 39% for selexipag and the active metabolite, respectively. Intra-subject variability in exposure was 24% and 19% for selexipag and the active metabolite, respectively.
Exposures to selexipag and the active metabolite at steady-state in PAH patients and healthy subjects were similar. The pharmacokinetics of selexipag and the active metabolite in PAH patients were not influenced by the severity of the disease and did not change with time.
Both in healthy subjects and PAH patients, after oral administration, exposure at steady-state to the active metabolite is approximately 3- to 4-fold that of selexipag. Exposure to the active metabolite is approximately 30% higher after oral administration compared to the same intravenous dose in healthy subjects.
Absorption
The absolute bioavailability of selexipag is approximately 49%. Upon oral administration, maximum observed plasma concentrations of selexipag and its active metabolite are reached within about 1–3 hours and 3–4 hours, respectively.
In the presence of food, the absorption of selexipag was prolonged resulting in a delayed time to peak concentration (Tmax) and ~30% lower peak plasma concentration (Cmax). The exposure to selexipag and the active metabolite (AUC) did not significantly change in the presence of food.
Distribution
The volume of distribution of selexipag at steady state is 11.7 L.
Selexipag and its active metabolite are highly bound to plasma proteins (approximately 99% in total and to the same extent to albumin and alpha1-acid glycoprotein).
Metabolism
Selexipag is hydrolyzed to its active metabolite, (free carboxylic acid) in the liver and intestine by carboxylesterases. Oxidative metabolism, catalyzed mainly by CYP2C8 and to a smaller extent by CYP3A4, leads to the formation of hydroxylated and dealkylated products. UGT1A3 and UGT2B7 are involved in the glucuronidation of the active metabolite. Except for the active metabolite, none of the circulating metabolites in human plasma exceeds 3% of the total drug-related material.
Elimination
Elimination of selexipag is predominately via metabolism with a mean terminal half-life of 0.8-2.5 hours. The terminal half-life of the active metabolite is 6.2-13.5 hours. There is minimal accumulation of the active metabolite upon twice daily repeat administration suggesting that the effective half-life is in the range of 3-4 hours. The total body clearance of selexipag is 17.9 L/hour.
Excretion
In a study in healthy subjects with radiolabeled selexipag, approximately 93% of radioactive drug material was eliminated in feces and only 12% in urine. Neither selexipag nor its active metabolite were found in urine.
Specific Populations:
No clinically relevant effects of sex, race, age or body weight on the pharmacokinetics of selexipag and its active metabolite have been observed in healthy subjects or PAH patients.
Age:
The pharmacokinetic variables (Cmax and AUC) were similar in adult and elderly subjects up to 75 years of age. There was no effect of age on the pharmacokinetics of selexipag and the active metabolite in PAH patients.
Hepatic Impairment:
In subjects with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, exposure to selexipag was 2- and 4-fold that seen in healthy subjects. Exposure to the active metabolite of selexipag remained almost unchanged in subjects with mild hepatic impairment and was doubled in subjects with moderate hepatic impairment [see Use in Specific Populations (8.6)].
Based on pharmacokinetic modeling of data from a study in subjects with hepatic impairment, the exposure to the active metabolite at steady state in subjects with moderate hepatic impairment (Child-Pugh class B) after a once daily regimen is expected to be similar to that in healthy subjects receiving a twice daily regimen. The exposure to selexipag at steady state in these patients during a once daily regimen is predicted to be approximately 2-fold that seen in healthy subjects receiving a twice-daily regimen.
Renal Impairment:
A 40-70% increase in exposure (maximum plasma concentration and area under the plasma concentration-time curve) to selexipag and its active metabolite was observed in subjects with severe renal impairment (estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2 and < 30 mL/min/1.73 m2) [see Use in Specific Populations (8.7)].
Drug Interaction Studies:
In vitro studies
Selexipag is hydrolyzed to its active metabolite by carboxylesterases. Selexipag and its active metabolite both undergo oxidative metabolism mainly by CYP2C8 and to a smaller extent by CYP3A4. The glucuronidation of the active metabolite is catalyzed by UGT1A3 and UGT2B7. Selexipag and its active metabolite are substrates of OATP1B1 and OATP1B3. Selexipag is a substrate of P-gp, and the active metabolite is a substrate of the transporter of breast cancer resistance protein (BCRP).
Selexipag and its active metabolite do not inhibit or induce cytochrome P450 enzymes and transport proteins at clinically relevant concentrations.
The results on in vivo drug interaction studies are presented in Figure 1 and 2.
Figure 1 Effect of Other Drugs on UPTRAVI and its Active Metabolite
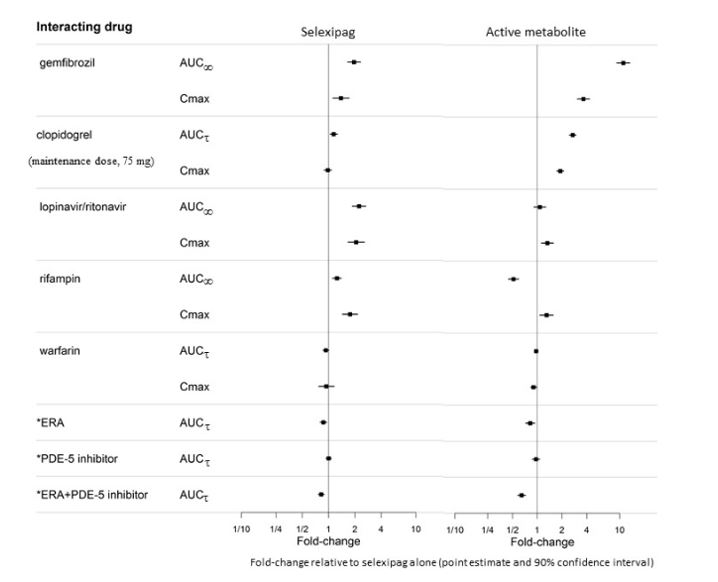
*ERA and PDE-5 inhibitor data from GRIPHON.
Figure 2 Effect of UPTRAVI on Other Drugs
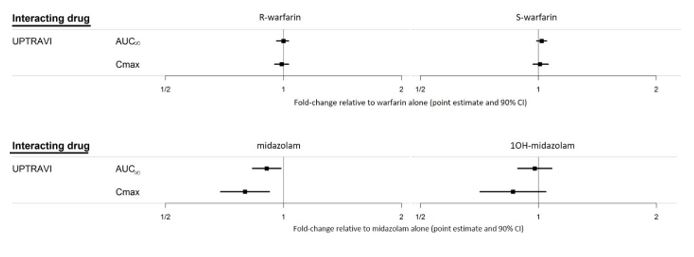
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: In the 2-year carcinogenicity studies, chronic oral administration of selexipag revealed no evidence of carcinogenic potential in rats at 100 mg/kg/day and mice at 500 mg/kg/day. The exposures were more than 25-fold human exposure.
-
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension
The effect of selexipag on progression of PAH was demonstrated in a multi-center, double-blind, placebo-controlled, parallel group, event-driven study (GRIPHON) in 1156 patients with symptomatic (WHO Functional Class I [0.8%], II [46%], III [53%], and IV [1%] ) PAH. Patients were randomized to either placebo (N = 582), or UPTRAVI (N = 574). The dose was increased in weekly intervals by increments of 200 mcg twice a day to the highest tolerated dose up to 1600 mcg twice a day.
The primary study endpoint was the time to first occurrence up to end-of-treatment of: a) death, b) hospitalization for PAH, c) PAH worsening resulting in need for lung transplantation, or balloon atrial septostomy, d) initiation of parenteral prostanoid therapy or chronic oxygen therapy, or e) other disease progression based on a 15% decrease from baseline in 6MWD plus worsening of Functional Class or need for additional PAH-specific therapy.
The mean age was 48 years, the majority of patients were white (65%) and female (80%). Nearly all patients were in WHO Functional Class II and III at baseline.
Idiopathic or heritable PAH was the most common etiology in the study population (58%) followed by PAH associated with connective tissue disease (29%), PAH associated with congenital heart disease with repaired shunts (10%), drugs and toxins (2%), and HIV (1%).
At baseline, the majority of enrolled patients (80%) were being treated with a stable dose of an endothelin receptor antagonist (15%), a PDE-5 inhibitor (32%), or both (33%).
Patients on selexipag achieved doses within the following groups: 200-400 mcg (23%), 600-1000 mcg (31%) and 1200-1600 mcg (43%).
Treatment with UPTRAVI resulted in a 40% reduction (99% CI: 22 to 54%; two-sided log-rank p-value < 0.0001) of the occurrence of primary endpoint events compared to placebo (Table 2; Figure 3). The beneficial effect of UPTRAVI was primarily attributable to a reduction in hospitalization for PAH and a reduction in other disease progression events (Table 2). The observed benefit of UPTRAVI was similar regardless of the dose achieved when patients were titrated to their highest tolerated dose [see Dosage and Administration (2.1)].
Figure 3 Kaplan-Meier Estimates of the First Morbidity-Mortality Event in GRIPHON
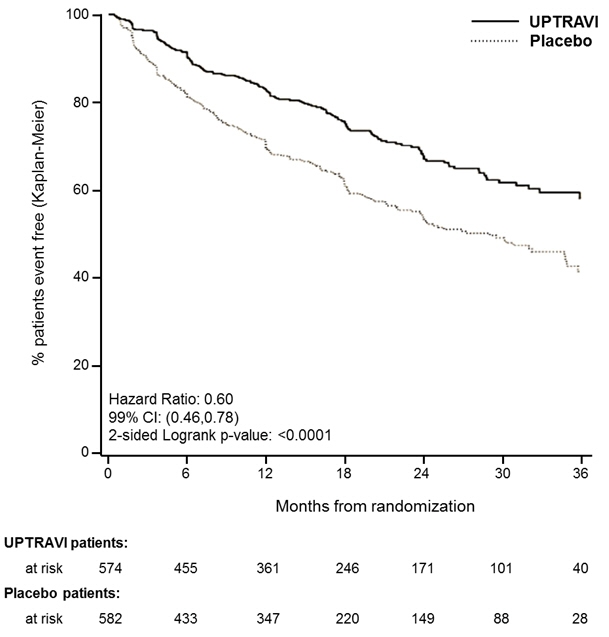
Table 2 Primary Endpoints and Related Components in GRIPHON UPTRAVI
N=574Placebo
N=582Hazard Ratio
(99% CI)p-value n % n % Primary endpoint events up to the end of treatment All primary endpoint events 155 27.0 242 41.6 0.60 [0.46,0.78] <0.0001 As first event: - Hospitalization for PAH
78 13.6 109 18.7 - Other disease Progression (Decrease in 6MWD plus worsening functional class or need for other therapy)
38 6.6 100 17.2 - Death
28 4.9 18 3.1 - Parenteral prostanoid or chronic oxygen therapy
10 1.7 13 2.2 - PAH worsening resulting in need for lung transplantation or balloon atrial septostomy
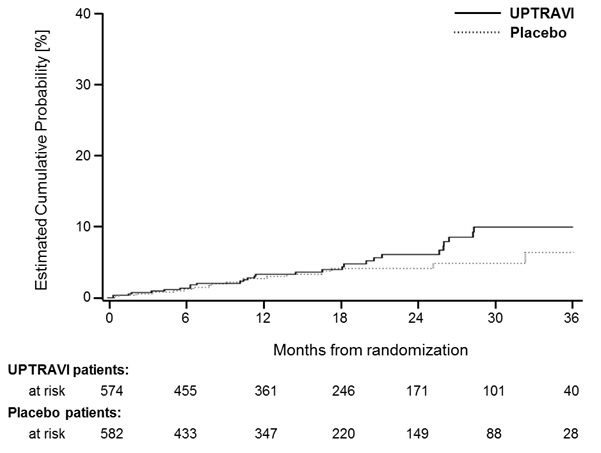
1 0.2 2 0.3 It is not known if the excess number of deaths in the selexipag group is drug-related because there were so few deaths and the imbalance was not observed until 18 months into GRIPHON.
Figures 4A, B, and C show time to first event analyses for primary endpoint components of hospitalization for PAH (A), other disease progression (B), and death (C)—all censored 7 days after any primary end point event (because many patients on placebo transitioned to open-label UPTRAVI at this point).
Figure 4 A Hospitalization for PAH as the First Endpoint in GRIPHON
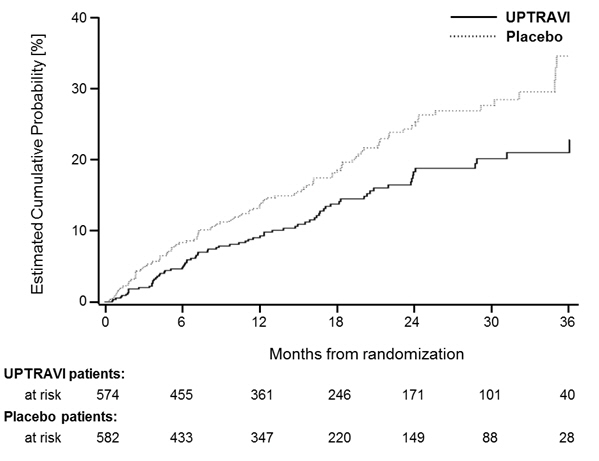
Figure 4B Disease Progression as the First Endpoint in GRIPHON
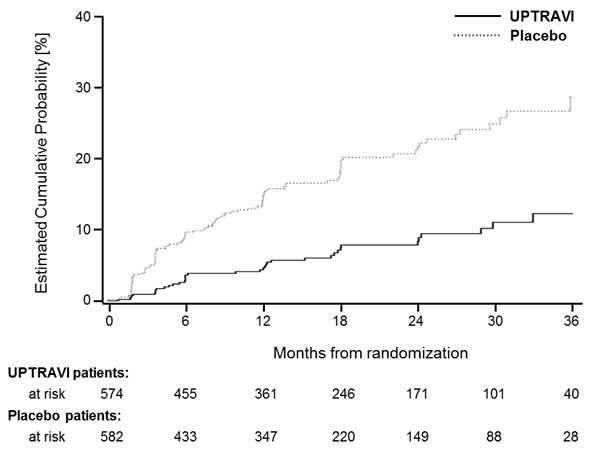
Figure 4C Death as the First Endpoint in GRIPHON
The treatment effect of UPTRAVI on time to first primary event was consistent irrespective of background PAH therapy (i.e., in combination with an ERA, PDE-5i, both, or without background therapy) (Figure 5).
Figure 5 Subgroup Analyses of the Primary Endpoint in GRIPHON
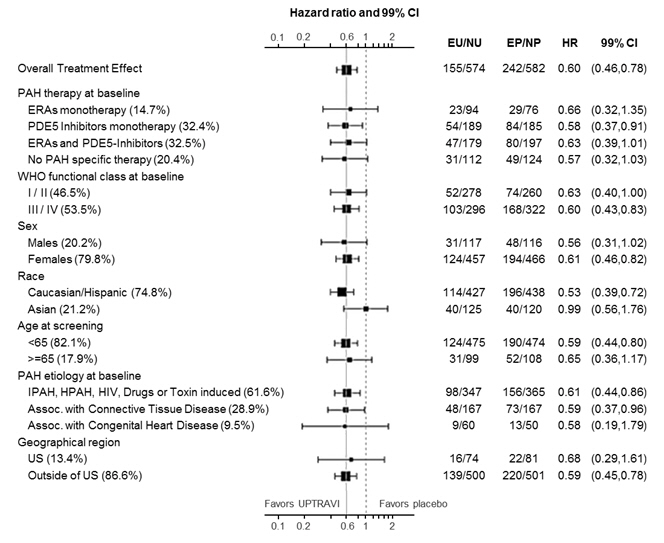
Note: Race group "Other" is not displayed in analysis, as the population is less than 30. EU = Number of UPTRAVI patients with events, NU = Number of patients randomized to UPTRAVI, EP = Number of Placebo patients with events, NP = Number of patients randomized to Placebo, HR = Hazard Ratio, CI = Confidence Interval, the size of the squares represent the number of patients in the subgroup.
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all were pre-specified. The 99% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
6-Minute Walk Distance (6MWD)
Exercise capacity was evaluated as a secondary endpoint. Median absolute change from baseline to week 26 in 6MWD measured at trough (i.e., at approximately 12 hours post-dose) was +4 meters with UPTRAVI and -9 meters in the placebo group. This resulted in a placebo-corrected median treatment effect of 12 meters (99% CI: 1, 24 meters;two-sided p = 0.005).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
UPTRAVI (selexipag) film-coated, round tablets are supplied in the following configurations:
Strength (mcg) Color Debossing NDC-XXX
Bottle of 60NDC-XXX
Bottle of 140200 Light yellow 2 66215-602-06 66215-602-14 400 Red 4 66215-604-06 Not Applicable 600 Light violet 6 66215-606-06 Not Applicable 800 Green 8 66215-608-06 Not Applicable 1000 Orange 10 66215-610-06 Not Applicable 1200 Dark violet 12 66215-612-06 Not Applicable 1400 Dark yellow 14 66215-614-06 Not Applicable 1600 Brown 16 66215-616-06 Not Applicable UPTRAVI is also supplied in a Titration Pack [NDC: 66215-628-20] that includes a 140 count bottle of 200 mcg tablets and a 60 count bottle of 800 mcg tablets.
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
UPTRAVI (up-TRA-vee)
(selexipag) tabletsThe Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 09/2019 Read this Patient Information before you start taking UPTRAVI and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. What is UPTRAVI? - UPTRAVI is a prescription medicine used to treat pulmonary arterial hypertension (PAH) which is high blood pressure in the arteries of your lungs.
- UPTRAVI can help slow down the progression of your disease and lower your risk of being hospitalized for PAH.
It is not known if UPTRAVI is safe and effective in children.Who should not take UPTRAVI? Do not take UPTRAVI if you
Take gemfibrozil because this medicine may affect how UPTRAVI works and cause side effects.What should I tell my healthcare provider before taking UPTRAVI?
Before you take UPTRAVI, tell your healthcare provider if you:- have liver problems.
- have narrowing of the pulmonary veins, a condition called pulmonary veno-occlusive disease.
- are pregnant or plan to become pregnant. It is not known if UPTRAVI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if UPTRAVI passes into your breast milk. You and your healthcare provider should decide if you will take UPTRAVI or breastfeed. You should not do both.
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. UPTRAVI and other medicines may affect each other causing side effects. Do not start any new medicine until you check with your healthcare provider.How should I take UPTRAVI? - Take UPTRAVI exactly as your healthcare provider tells you to take it. Do not stop taking UPTRAVI unless your healthcare provider tells you to stop.
- Your healthcare provider will slowly increase your dose to find the dose of UPTRAVI that is right for you.
- If you have side effects, your healthcare provider may tell you to change your dose of UPTRAVI.
- UPTRAVI can be taken with or without food. Taking UPTRAVI with food may help you tolerate UPTRAVI better.
- UPTRAVI is usually taken 2 times each day.
- Swallow UPTRAVI tablets whole. Do not split, crush or chew UPTRAVI tablets.
- If you miss a dose of UPTRAVI, take it as soon as you remember. If your next scheduled dose is due within 6 hours, skip the missed dose. Take the next dose at your regular time.
- If you miss 3 or more days of UPTRAVI, call your healthcare provider to see if your dose needs to be changed.
- If you take too much UPTRAVI, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of UPTRAVI?
The most common side effects of UPTRAVI include:- Headache
- jaw pain
- muscle pain
- pain in arms or legs
- pain in joints
- decreased appetite
- diarrhea
- nausea
- vomiting
- flushing
- low red blood cell count
- rash
These are not all of the possible side effects of UPTRAVI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store UPTRAVI? - Store UPTRAVI tablets at room temperature between 68°F and 77°F (20°C and 25°C).
Keep UPTRAVI and all medicines out of the reach of children.General information about the safe and effective use of UPTRAVI
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
Do not use UPTRAVI for a condition for which it was not prescribed. Do not give UPTRAVI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about UPTRAVI that is written for health professionals.What are the ingredients in UPTRAVI?
Active ingredient: selexipag
Inactive ingredients: D-mannitol, corn starch, low substituted hydroxypropylcellulose, hydroxypropylcellulose, and magnesium stearate. The tablets are film coated with a coating material containing hypromellose, propylene glycol, titanium dioxide, carnauba wax along with iron oxide red, iron oxide yellow, or iron oxide black.
Manufactured for: Actelion Pharmaceutical US, Inc. 5000 Shoreline Court, Ste. 200 South San Francisco, CA 94080, USA
ACT20190806
©2017 Actelion Pharmaceuticals US, Inc. All rights reserved.
For more information, call 1-866-228-3546 or go to www.UPTRAVI.com. - PRINCIPAL DISPLAY PANEL - 200 mcg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 400 mcg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 600 mcg Tablet Bottle Carton
- PRINCIPAL DISPLAY PANEL - 800 mcg Tablet Bottle Carton
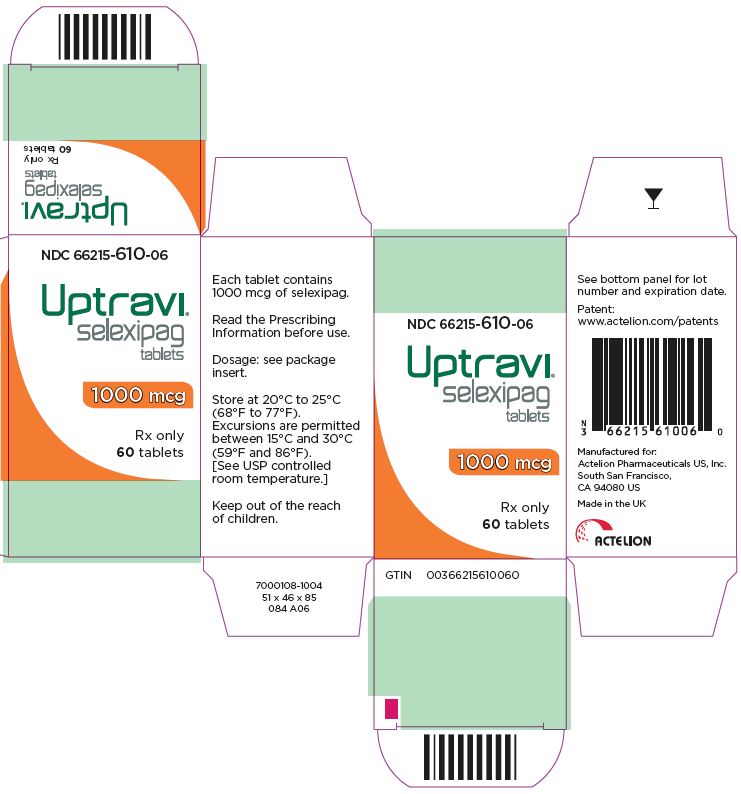
- PRINCIPAL DISPLAY PANEL - 1000 mcg Tablet Bottle Carton
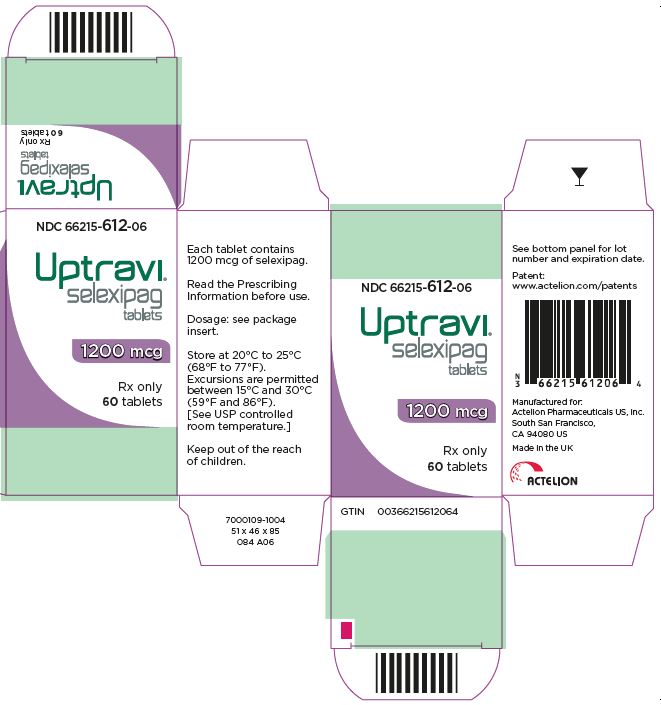
- PRINCIPAL DISPLAY PANEL - 1200 mcg Tablet Bottle Carton
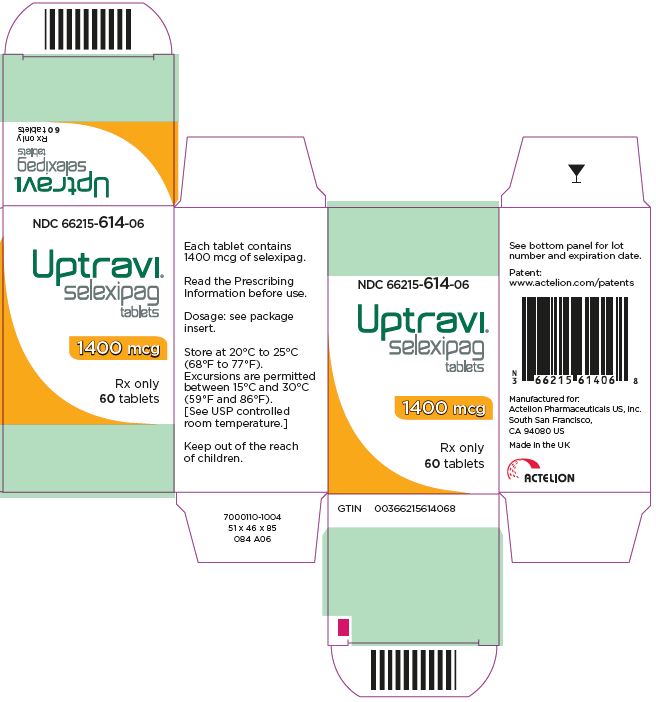
- PRINCIPAL DISPLAY PANEL - 1400 mcg Tablet Bottle Carton
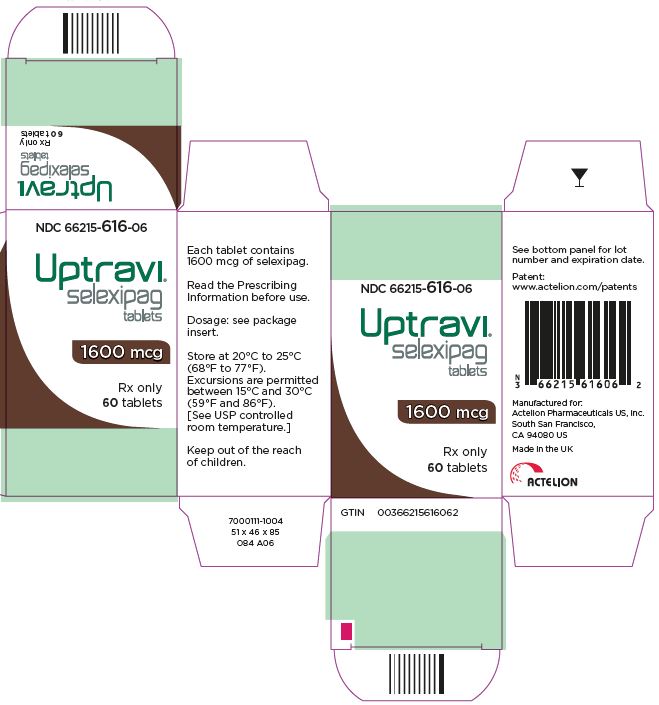
- PRINCIPAL DISPLAY PANEL - 1600 mcg Tablet Bottle Carton
-
PRINCIPAL DISPLAY PANEL - Kit Carton
NDC: 66215-628-20
TITRATION PACK
Uptravi®
selexipag
tablets200 mcg
Rx only
140 tabletsUptravi®
selexipag
tablets800 mcg
Rx only
60 tablets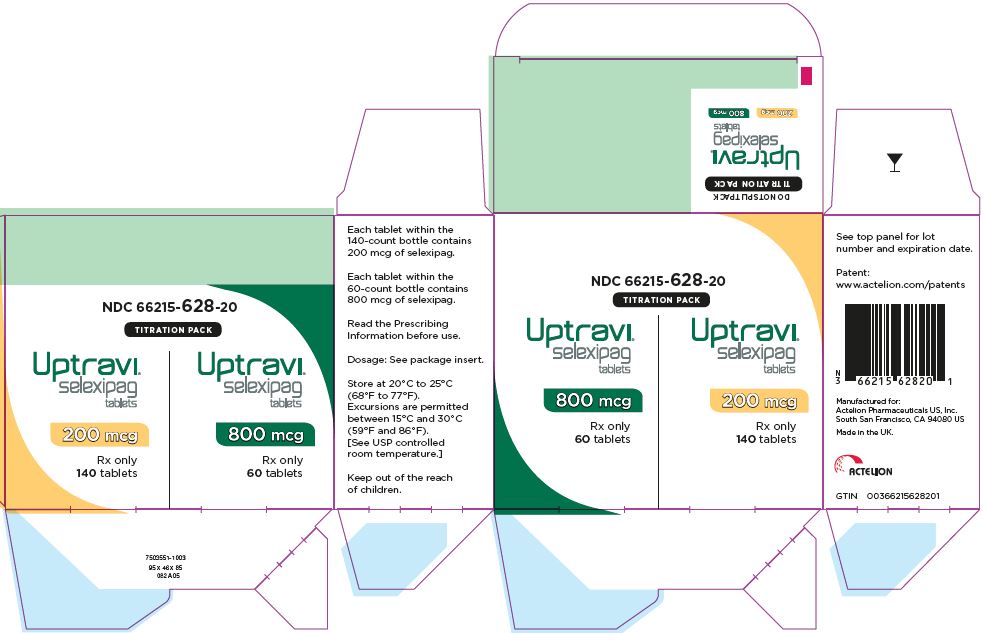
-
INGREDIENTS AND APPEARANCE
UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 200 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color YELLOW (light yellow) Score no score Shape ROUND Size 7mm Flavor Imprint Code 2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-602-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 66215-602-14 1 in 1 CARTON 12/21/2015 2 140 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-604 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 400 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color RED (red) Score no score Shape ROUND Size 7mm Flavor Imprint Code 4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-604-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-606 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 600 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color PURPLE (light violet) Score no score Shape ROUND Size 7mm Flavor Imprint Code 6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-606-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-608 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 800 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color GREEN (green) Score no score Shape ROUND Size 7mm Flavor Imprint Code 8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-608-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-610 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 1000 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color ORANGE (orange) Score no score Shape ROUND Size 7mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-610-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-612 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 1200 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color PURPLE (dark violet) Score no score Shape ROUND Size 7mm Flavor Imprint Code 12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-612-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-614 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 1400 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color YELLOW (dark yellow) Score no score Shape ROUND Size 7mm Flavor Imprint Code 14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-614-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI
selexipag tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-616 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 1600 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color BROWN (brown) Score no score Shape ROUND Size 7mm Flavor Imprint Code 16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-616-06 1 in 1 CARTON 12/21/2015 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 UPTRAVI TITRATION PACK
selexipag kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-628 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-628-20 1 in 1 CARTON 12/21/2015 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 140 Part 2 1 BOTTLE 60 Part 1 of 2 UPTRAVI
selexipag tablet, coatedProduct Information Item Code (Source) NDC: 66215-602 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 200 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color YELLOW (light yellow) Score no score Shape ROUND Size 7mm Flavor Imprint Code 2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 140 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 Part 2 of 2 UPTRAVI
selexipag tablet, coatedProduct Information Item Code (Source) NDC: 66215-608 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELEXIPAG (UNII: 5EXC0E384L) (SELEXIPAG - UNII:5EXC0E384L) SELEXIPAG 800 ug Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 2165RE0K14) HYDROXYPROPYL CELLULOSE (1200000 WAMW) (UNII: U3JF91U133) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color GREEN (green) Score no score Shape ROUND Size 7mm Flavor Imprint Code 8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207947 12/21/2015 Labeler - Actelion Pharmaceuticals US, Inc. (002641228)
Trademark Results [UPTRAVI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 UPTRAVI 86829111 5074971 Live/Registered |
Actelion Pharmaceuticals Ltd 2015-11-23 |
 UPTRAVI 85348761 4087965 Live/Registered |
Actelion Pharmaceuticals Ltd 2011-06-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.