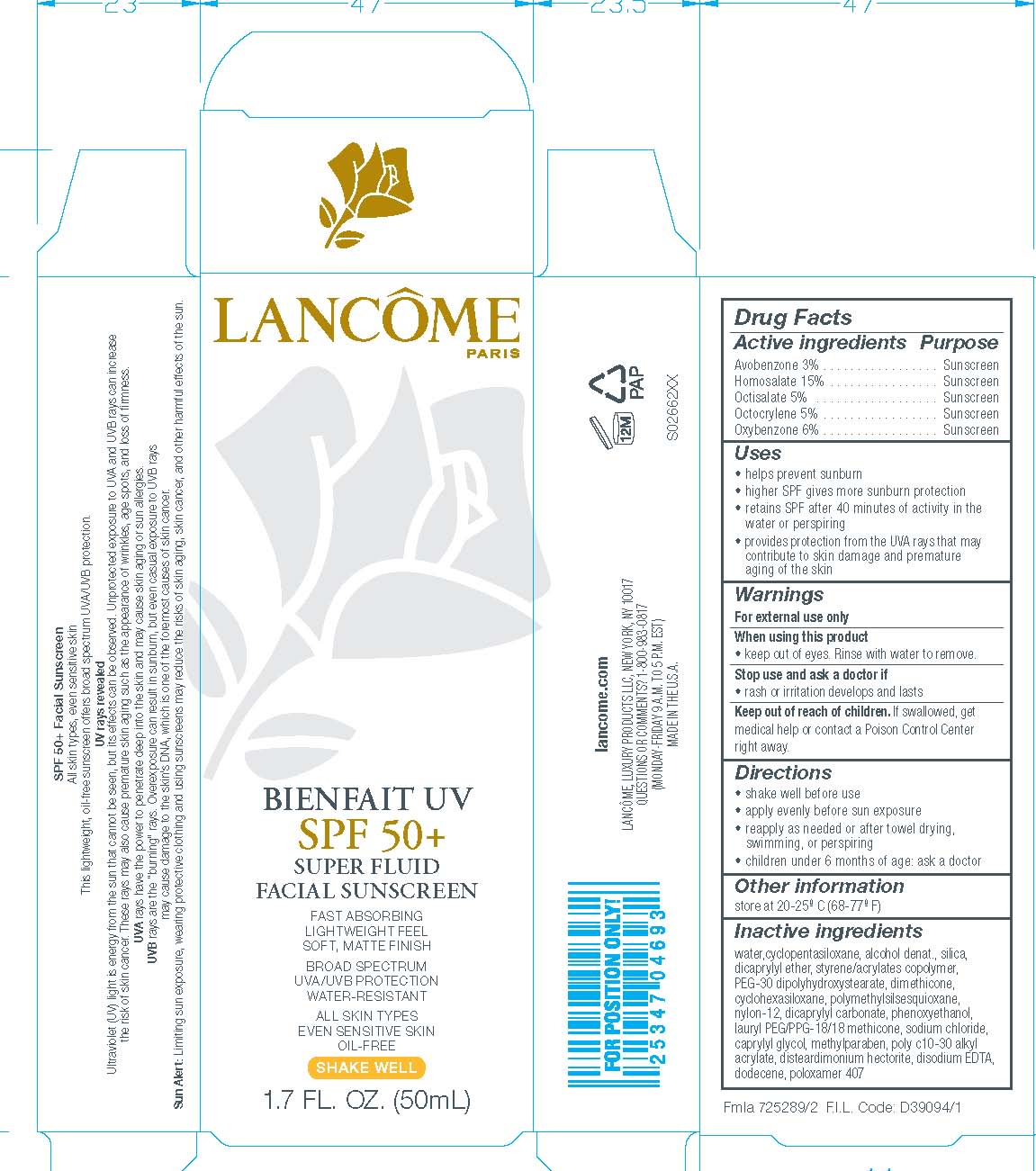
LANCOME BIENFAIT UV SPF 50 PLUS SUPER FLUID FACIAL SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene and oxybenzone lotion
L'Oreal USA Products Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Avobenzone 3%
Homosalate 15%
Octisalate 5%
Octocrylene 5%
Oxybenzone 6%
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
- retains SPF after 40 minutes of activity in the water or perspiring
- provides protection from the UVA rays that may contribute to skin damage and premature aging of the skin
Directions
- shake well before use
- apply evenly before sun exposure
- reapply as needed or after towel drying, swimming, or perspiring
- children under 6 months of age: ask a doctor
Warnings
For external use only
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
rash or irritation develops and lasts
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Other information
Other information
store at 20-25o C (68-77oF)
Inactive ingredients
WATER
CYCLOPENTASILOXANE
ALCOHOL DENAT.
SILICA
DICAPRYLYL ETHER
STYRENE/ACRYLATES COPOLYMER
PEG-30 DIPOLYHYDROXYSTEARATE
DIMETHICONE
CYCLOHEXASILOXANE
POLYMETHYLSILSESQUIOXANE
NYLON-12
DICAPRYLYL CARBONATE
PHENOXYETHANOL
LAURYL PEG/PPG-18/18 METHICONE
SODIUM CHLORIDE
CAPRYLYL GLYCOL
METHYLPARABEN
POLY C10-30 ALKYL ACRYLATE
DISTEARDIMONIUM HECTORITE
DISODIUM EDTA
DODECENE
POLOXAMER 407