K-PURE by NIBEC Co., Ltd.
K-PURE by
Drug Labeling and Warnings
K-PURE by is a Otc medication manufactured, distributed, or labeled by NIBEC Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
K-PURE- alcohol gel
NIBEC Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
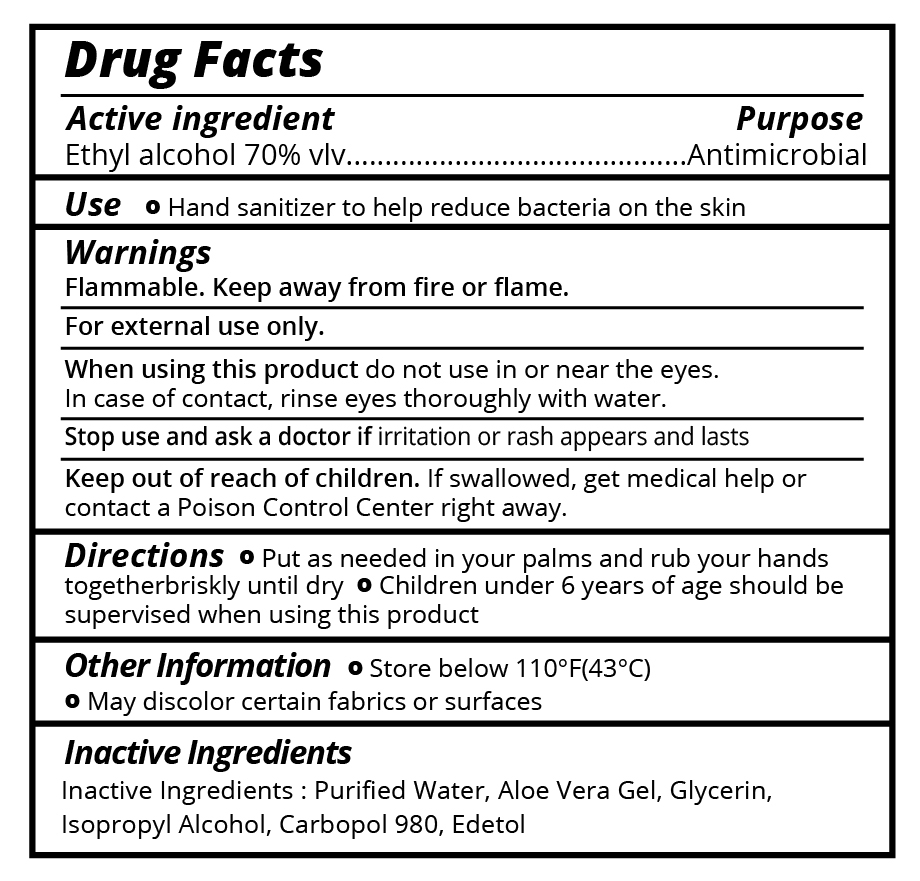
Flammable. Keep away from fire or flame.
For external use only.
When using this productdo not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Stop use and ask a doctorif irritation or rash appears and lasts.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
| K-PURE
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - NIBEC Co., Ltd. (687796909) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NIBEC Co., Ltd. | 687796909 | manufacture(47649-0003) | |
Revised: 9/2023
Document Id: 05484381-1ecd-388b-e063-6394a90aa378
Set id: a7b4e528-fe49-6db6-e053-2995a90af198
Version: 3
Effective Time: 20230913
Trademark Results [K-PURE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 K-PURE 85171900 4560577 Live/Registered |
INNOPHOS NUTRITION, INC. 2010-11-08 |
 K-PURE 78283220 2877517 Live/Registered |
King Industries, Inc. 2003-08-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.