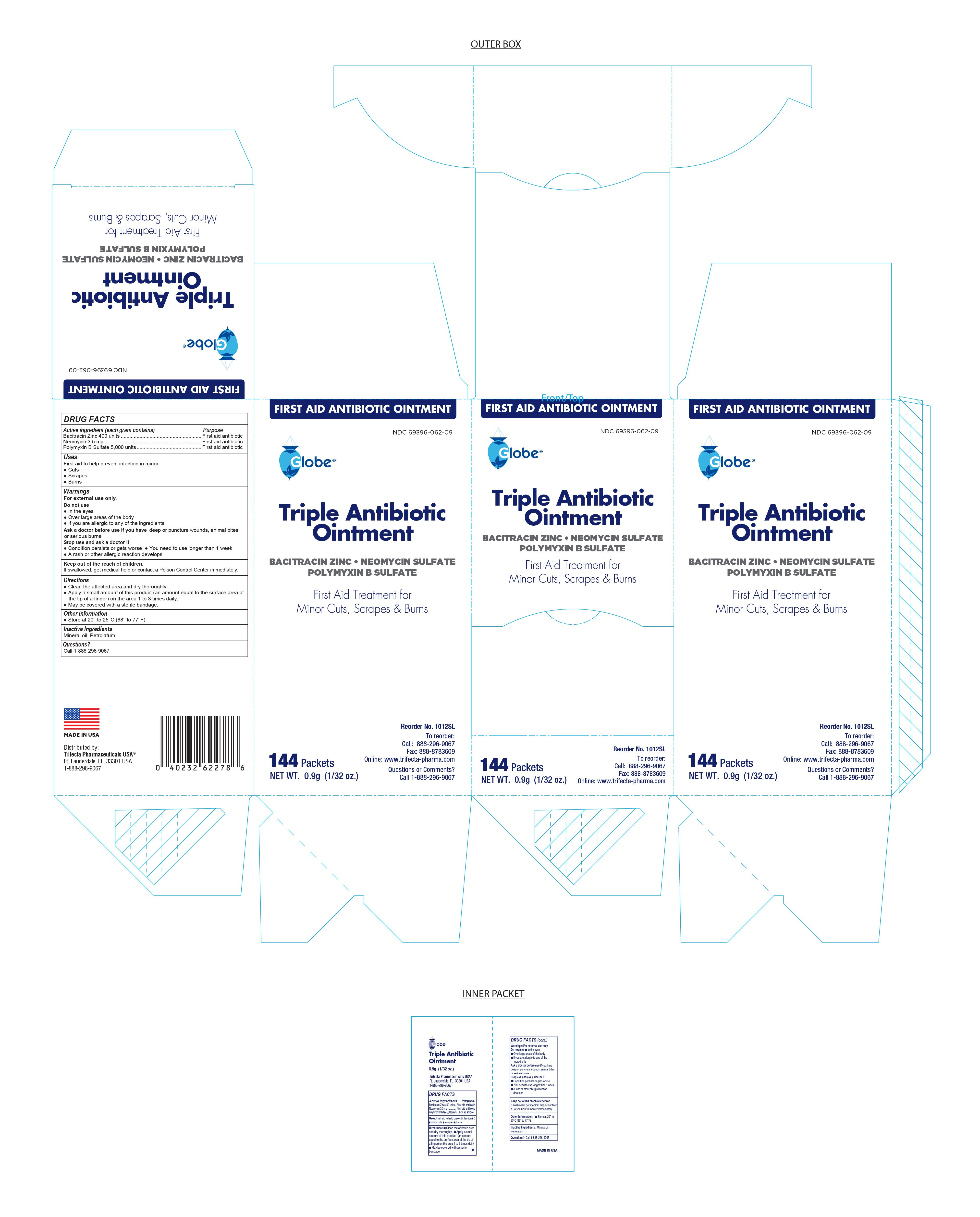
Globe Triple Antibiotic Ointment
Triple Antibiotic by
Drug Labeling and Warnings
Triple Antibiotic by is a Otc medication manufactured, distributed, or labeled by Trifecta Pharmaceuticals USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment
Trifecta Pharmaceuticals USA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Globe Triple Antibiotic Ointment
Stop Use and Ask Doctor if
- If you need to use longer than 1 week
- The condition persists or gets worse
- A rash or allergic reaction develops
Directions
- Clean the affected area and dry thoroughly
- Apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- May be covered with a sterile bandage
Questions
Call 1-888-296-9067
Fax: 1-888-878-3609
Online: www.trifecta-pharma.com
Reorder No. 1012SL
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center immediately.
| TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Trifecta Pharmaceuticals USA LLC (079424163) |
Revised: 7/2023
Document Id: ffc092b2-b835-c91b-e053-6394a90a3a06
Set id: a7bfa4b5-72b7-334a-e053-2a95a90aed3d
Version: 3
Effective Time: 20230705