Crosstex SaniTyze Hand Sanitizer
SaniTyze by
Drug Labeling and Warnings
SaniTyze by is a Otc medication manufactured, distributed, or labeled by Crosstex International Inc., Inopak, Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANITYZE- hand sanitizer gel
Crosstex International Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crosstex SaniTyze Hand Sanitizer
Keep away from Children
Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Uses
To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings
Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Stop Use
Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours.
Inactive Ingredients
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
Sanityze 2 oz
Front
SaniTyze™
HAND SANITIZER
With
Aloe Vera & Vitamin E
Developed for
Healthcare Professionals
2 fl oz (60 ml)
CROSSTEX
A CANTEL MEDICAL COMPANY
Back
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ MADE IN JWG2-BTL
HAND SANITIZER USA Rev.3-9/18
REF JWG2
|
Bar code |
(01)00732224002767
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com


SaniTyze 4 oz
Font
SaniTyze™
HAND SANITIZER
With
Aloe Vera & Vitamin E
Developed for
Healthcare Professionals
4 fl oz (118 ml)
CROSSTEX
A CANTEL MEDICAL COMPANY
Back
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ MADE IN JWG-BTL
HAND SANITIZER USA Rev.3-9/18
REF JWG
|
Bar code |
(01)00732224002774
MADE IN
USA
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com


SaniTyze 8 oz
Front
SaniTyze™
HAND SANITIZER
With
Aloe Vera & Vitamin E
Developed for
Healthcare Professionals
8 fl oz (237 ml)
CROSSTEX
A CANTEL MEDICAL COMPANY
Back label
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ REF JWG
|
Bar code |
HAND SANITIZER
JWG8-BTL
MADE IN JWG8 (01) 00732224002743
Rev. 3- 9/18
MADE IN
USA
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com


SaniTyze 18 oz
Front
SaniTyze™
HAND SANITIZER
With
Aloe Vera & Vitamin E
Developed for
Healthcare Professionals
18 fl oz (532 ml)
CROSSTEX
A CANTEL MEDICAL COMPANY
Back 18 oz
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ REF JWGP
HAND SANITIZER
JWGP-BTL BAR CODE
MADE IN JWG8 (01) 00732224002750
Rev. 3- 9/18
MADE IN
USA
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com


SaniTyze 28.2 oz
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ REF JWGEL
HAND SANITIZER
28.2 fl oz (800ml) BAR CODE
MADE IN JWG8 (01) 00732224002736
MADE IN
USA
CROSSTEX®
A CANTEL MEDICAL COMPANY
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA JWGEL-PK
Website: www.crosstex.com E-mail: crosstex@crosstex.com Rev. 3- 9/18

SaniTyze 33.8 oz
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% v/v …………….. Antiseptic
Uses: To decrease bacteria on the skin that potentially can cause disease. Recommended for repeated use
Warnings: Flammable, keep away from heat or flame. For external use only. Keep out of eyes, ears or mouth. In case of eye contact, flush eyes with water. Stop use and ask a doctor if irritation and redness develop or if condition persists for more than 72 hours. Keep out of reach of children. If swallowed. Get medical help or contact a Poison Control Center right away. Children should be supervised by an adult when using this product.
Directions: Apply sufficient amount of product to your palm to cover both hands. Rub until dry.
Other Information: May discolor certain fabrics and surfaces.
Inactive Ingredients:
Acrylates/C 10/30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, FD&C Blue 1, FD&C Yellow 5, Fragrance, Isopropyl Alcohol, PEG/PPG-18/18 Dimethicone, Triethanolamine, Vitamin E, Water
SaniTyze™ REF JWGEL1
HAND SANITIZER
33.8 fl oz (1000ml) BAR CODE
PWGEL1-PK (01) 00732224002729
Rev. 3- 9/18
MADE IN
USA
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com
CROSSTEX®
A CANTEL MEDICAL COMPANY

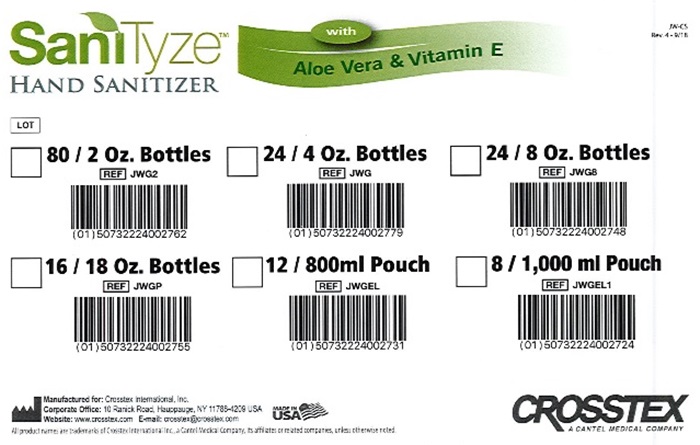
SaniTyze Case label
SaniTyze™ with JW-CS
HAND SANITIZER Aloe Vera & Vitamin E Rev. 4- 9/18
|
LOT |
80 / 2 Oz. Bottles 24 / 4 Oz. Bottles 24 / 8 Oz. Bottles
JWG2 JWG JWG8
|
Bar code |
Bar code |
Bar code |
(01)00732224002762 (01)00732224002779 (01)00732224002748
16 / 18 Oz. Bottles 12 / 800ml Pouch 8 / 1,000 ml Pouch
JWGP JWGEL JWGEL1
|
Bar code |
Bar code |
Bar code |
(01)00732224002755 (01)00732224002731 (01)00732224002724
Manufactured for Crosstex International Inc.
Corporate Office 10 Ranch Road Hauppauge NY 11788-4209 USA
Website: www.crosstex.com E-mail: crosstex@crosstex.com
MADE IN
USA
CROSSTEX®
A CANTEL MEDICAL COMPANY
| SANITYZE
hand sanitizer gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Crosstex International Inc. (057728685) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Crosstex International Inc. | 057728685 | label(24794-138) | |