TUXARIN- codeine phosphate and chlorpheniramine maleate tablet, extended release
Tuxarin by
Drug Labeling and Warnings
Tuxarin by is a Prescription medication manufactured, distributed, or labeled by Mainpointe Pharmaceuticals, LGM Pharma Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TUXARIN ER™ safely and effectively. See full prescribing information for TUXARIN ER.
TUXARIN ER (codeine phosphate and chlorpheniramine maleate) extended release tablets, CIII
Initial U.S. Approval: 1985WARNING ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
See full prescribing information for complete boxed warning.
- Life threatening respiratory depression and death have occurred in children who received codeine; most cases followed tonsillectomy and/or adenoidectomy and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a CYP2D6 polymorphism. [See Warnings and Precautions (5.1)]. TUXARIN ER is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [See Contraindications(4)]. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. [See Warnings and Precautions (5.1)].
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warning and Precautions (5.2) Drug Interactions (7.1)]. Avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol.
RECENT MAJOR CHANGES
Boxed Warning 8/2017 Contraindications (4) 8/2017 Warnings and Precautions (5.1) 8/2017 Warnings and Precautions (5.2) 1/2017 INDICATIONS AND USAGE
TUXARIN ER is a combination of codeine phosphate, an opiate agonist antitussive, and chlorpheniramine maleate, a histamine-1 (H1) receptor antagonist indicated for the relief of cough and symptoms associated with upper respiratory allergies or a common cold. (1) Important Limitations of Use Not indicated for pediatric patients under 18 years of age (8.4))
DOSAGE AND ADMINISTRATION
Adults and children 18 years of age and older: 1 tablet every 12 hours, not to exceed 2 doses in 24 hours. (2)
DOSAGE FORMS AND STRENGTHS
Extended release (ER) tablet: contains 54.3 mg of codeine phosphate (equivalent to 40 mg of codeine) and 8 mg of chlorpheniramine maleate (equivalent to 5.6 mg of chlorpheniramine). (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of death in ultra-rapid metabolizers: Conversion of codeine into its active metabolite, morphine, may occur more rapidly and completely resulting in higher than expected morphine levels and respiratory depression or death. (5.1)
- Risks using with Benzodiazepines or other CNS Depressants. (5.2)
- Dose-related respiratory depression. Use with caution. (5.3)
- Drug dependence: Prescribe with caution that is appropriate to the use of other opioids. (5.4)
- Head injury, intra-cranial lesions, or increased intracranial pressure: Avoid in patients with head injury, intra-cranial lesions, or increased intracranial pressure. (5.5)
- Activities requiring mental alertness: Avoid engaging in hazardous tasks requiring complete mental alertness such as driving or operating machinery. Avoid concurrent use of alcohol or other central nervous system depressants. (5.6)
- Prolonged use may cause Obstructive Bowel Disease (5.7)
- Acute abdominal conditions: Use caution in patients with acute abdominal conditions. (5.8)
- Special risk patients: Caution in elderly patients and those with asthma, persistent or chronic cough, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. (5.9)
ADVERSE REACTIONS
Common adverse reactions of TUXARIN ER include: Nausea and vomiting, constipation, abdominal distension, abdominal pain, blurred vision, diplopia, visual disturbances, confusion, dizziness, depression, drowsiness, sedation, headache, euphoria, facial dyskinesia, feeling faint, light-headedness, general feeling of discomfort or illness, excitability, nervousness, agitation, restlessness, somnolence, insomnia, dyskinesia, irritability, tremor. (6)
To report SUSPECTED ADVERSE REACTIONS, contact MainPointe Pharmaceuticals, LLC at 502-709-7544 or go to mainpointepharmaceuticals.com or FDA at 1-800-FDA-1088
DRUG INTERACTIONS
- Opioids, antihistamines, antipsychotics, anti-anxiety agents, or other CNS depressants: may cause additive CNS depression. (7.1)
- MAOIs or tricyclic antidepressants: may increase the effect of either the antidepressant or codeine. (7.2)
- Anticholinergic drugs: Use with caution. Additive adverse effects resulting from cholinergic blockage (e.g., xerostomia, blurred vision, or constipation) may occur. (7.3)
- Inhibitors or inducers of metabolic enzymes: Concomitant use of cytochrome P450 2D6 and 3A4 enzyme inhibitors or inducers may result in an altered response to codeine, monitor antitussive activity. Chlorpheniramine may inhibit the hepatic metabolism of phenytoin, monitor phenytoin toxicity. (7.4)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Labor: Use of codeine during labor can produce respiratory depression in the neonate. (8.2)
- Lactation: Breastfeeding not recommended. (8.3)
- Pediatric patients: Safety and effectiveness of this drug product has not been established for patients under 18. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN And RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults 18 Years of Age and Older
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children
5.2 Risks from Concomitant Use with Benzodiazepines or other CNS Depressants
5.3 Respiratory Depression
5.4 Drug Dependence
5.5 Head Injury and Increased Intracranial Pressure
5.6 Activities Requiring Mental Alertness
5.7 Obstructive Bowel Disease
5.8 Acute Abdominal Conditions
5.9 Special Risk Patients
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Benzodiazepines, Opioids, Antihistamines, Antipsychotics, Anti-anxiety Agents, or Other CNS Depressants (Including Alcohol)
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
7.3 Anticholinergic Drugs
7.4 Inhibitors or Inducers of Metabolic Enzymes
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN And RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children
Life threatening respiratory depression and death have occurred in children who received codeine; most cases followed tonsillectomy and/or adenoidectomy and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a CYP2D6 polymorphism. [See Warnings and Precautions (5.1)]. TUXARIN ER is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [See Contraindications(4)]. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. [See Warnings and Precautions (5.1)].
Concomitant Use with Benzodiazepines, CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warning and Precautions (5.2) Drug Interactions (7.1)]. Avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol.
-
1 INDICATIONS AND USAGE
TUXARIN ER is indicated for the relief of cough and symptoms associated with upper respiratory allergies or a common cold in adults 18 years of age and older.
Important Limitations of Use
Not indicated for pediatric patients under 18 years of age [see Use in Special Population (8.4)]
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- TUXARIN ER is contraindicated for:
- All children younger than 12 years of age [see Warnings and Precautions (5.1)].
- Postoperative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Warnings and Precautions (5.1)].
- Patients with known hypersensitivity to codeine, chlorpheniramine or any of the inactive ingredients of TUXARIN ER. Persons known to be hypersensitive to certain other opioids may exhibit cross-sensitivity to codeine.
-
5 WARNINGS AND PRECAUTIONS
5.1 Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received codeine. Codeine is subject to variability in metabolism based upon CYP2D6 genotype (described below), which can lead to an increased exposure to the active metabolite morphine. Based upon post-marketing reports, children less than 12 years old appear to be more susceptible to the respiratory depressant effects of codeine, particularly if there are risk factors for respiratory depression. For example, many reported cases of death occurred in the post-operative period following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being ultra-rapid metabolizers of codeine. Furthermore, children with obstructive sleep apnea who are treated with codeine for post-tonsillectomy and/or adenoidectomy pain may be particularly sensitive to its respiratory depressant effect. Because of the risk of life-threatening respiratory depression and death:
- TUXARIN ER is contraindicated in all children younger than 12 years of age [see Contraindications (4)].
- TUXARIN ER is contraindicated for post-operative management in pediatric patients younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)].
- Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. Risk factors include conditions associated with hypoventilation, such as postoperative status, obstructive sleep apnea, obesity, severe pulmonary disease, neuromuscular disease, and concomitant use of other medications that cause respiratory depression.
- When prescribing codeine for adolescents, healthcare providers should choose the lowest effective dose for the shortest period of time and inform patients and caregivers about these risks and the signs of morphine overdose [see Overdosage (10)].
Nursing Mothers
At least one death was reported in a nursing infant who was exposed to high levels of morphine in breast milk because the mother was an ultra-rapid metabolizer of codeine. Breastfeeding is not recommended during treatment with TUXARIN ER [see Use in Specific Populations (8.3)].
CYP2D6 Genetic Variability: Ultra-rapid metabolizer
Some individuals may be ultra-rapid metabolizers because of a specific CYP2D6 genotype (e.g., gene duplications denoted as *1/*1×N or *1/*2×N). The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 1 to 10% for Whites (European, North American), 3 to 4% for Blacks (African Americans), 1 to 2% for East Asians (Chinese, Japanese, Korean), and may be greater than 10% in certain ethnic groups (i.e., Oceanian, Northern African, Middle Eastern, Ashkenazi Jews, Puerto Rican). These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels.
Even at labeled dosage regimens, individuals who are ultra-rapid metabolizers may have life-threatening or fatal respiratory depression or experience signs of overdose (such as extreme sleepiness, confusion, or shallow breathing) [see Overdosage (10)] . Therefore, individuals who are ultra-rapid metabolizers should not use TUXARIN ER.
5.2 Risks from Concomitant Use with Benzodiazepines or other CNS Depressants
Concomitant use of opioids, including TUXARIN ER, with benzodiazepines, or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Because of these risks, avoid use of opioid cough medications in patients taking Benzodiazepines, other CNS depressants, or alcohol [see Drug Interactions (7.1)].
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. Because of similar pharmacologic properties, it is reasonable to expect similar risk with concomitant use of opioid cough medications and benzodiazepines, other CNS depressants, or alcohol.
Advise both patients and caregivers about the risks of respiratory depression and sedation if TUXARIN ER is used with benzodiazepines, alcohol, or other CNS depressants. [see Patient Counseling Information (17)]
5.3 Respiratory Depression
Codeine, one of the active ingredients in TUXARIN ER, produces dose-related respiratory depression by directly acting on brain stem respiratory centers.
Overdose of codeine in adults has been associated with fatal respiratory depression, and the use of codeine in children has been associated with fatal respiratory depression. Exercise caution when administering TUXARIN ER because of the potential for respiratory depression. If respiratory depression occurs, discontinue TUXARIN ER and use naloxone hydrochloride when indicated to antagonize the effect and other supportive measures as necessary. [see Overdosage (10)].
5.4 Drug Dependence
Codeine can produce drug dependence of the morphine type and, therefore, has the potential for being abused. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of TUXARIN ER. Prescribe and administer TUXARIN ER with the same degree of caution appropriate to the use of other opioid drugs. [see Drug Abuse and Dependence (9.2, 9.3)]
5.5 Head Injury and Increased Intracranial Pressure
The respiratory depression effects of opioids and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increase in intracranial pressure. Furthermore, opioids produce adverse reactions that may obscure the clinical course of patients with head injuries. The use of TUXARIN ER should be avoided in these patients.
5.6 Activities Requiring Mental Alertness
Codeine and chlorpheniramine, the active ingredients in TUXARIN ER, may produce marked drowsiness and impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Advise patients to avoid engaging in hazardous tasks requiring mental alertness and motor coordination after ingestion of TUXARIN ER. Concurrent use of TUXARIN ER with alcohol or other central nervous system depressants should be avoided because additional impairment of central nervous system performance may occur.
5.7 Obstructive Bowel Disease
Chronic use of opioids, including codeine, may result in obstructive bowel disease especially in patients with underlying intestinal motility disorders. Codeine may cause or aggravate constipation. Use with caution in patients with underlying intestinal motility disorders.
5.8 Acute Abdominal Conditions
TUXARIN ER should be used with caution in patients with acute abdominal conditions since the administration of codeine may obscure the diagnosis or clinical course of patients with acute abdominal conditions. The concurrent use of other anticholinergics with codeine may produce paralytic ileus. [see Drug Interactions (7.3)]
5.9 Special Risk Patients
As with other opioids, TUXARIN ER should be used with caution in elderly or debilitated patients and those with asthma, persistent or chronic cough, hypothyroidism, Addison's disease, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
-
6 ADVERSE REACTIONS
Use of codeine, an opioid, may result in the following:
- Respiratory depression [see Warnings and Precautions (5.1); (5.3) and Overdosage (10)]
- Drug dependence [see Warnings and Precautions (5.4)]
- Increased intracranial pressure [see Warnings and Precautions (5.5)]
- Decreased mental alertness with impaired mental and/or physical abilities [see Warnings and Precautions (5.6)]
- Paralytic ileus [see Warnings and Precautions (5.7)]
Use of chlorpheniramine, an antihistamine, may result in:
- Decreased mental alertness with impaired mental and/or physical abilities [see Warnings and Precautions (5.6)]
Adverse reactions listed below have been reported in the literature for codeine and chlorpheniramine and may be expected to occur with TUXARIN ER. Also included are events that occurred during clinical pharmacokinetic studies (in a total of 66 healthy adult volunteers with either single or multiple dose exposure) with TUXARIN ER and judged by the investigator to be related to study treatment. Because these reactions may be reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergic: Allergic laryngospasm, nasal stuffiness, bronchospastic allergic reaction, hives, itching, swelling of face.
Body as a whole: Asthenia, feeling of relaxation, redness or flushing of the face, unusual tiredness, weakness.
Cardiovascular: Fast, or slow heartbeat, hypertension, hypotension, orthostatic hypotension, palpitations, shock-like state, syncope.
Dermatological System: Skin rash, pruritus, erythema, urticaria, excessive perspiration, dermatitis.
Endocrine System: Changes in glucose utilization, decreased lactation, early menses, glycosuria, gynecomastia, hypoglycemia, increased appetite, increased libido, pheochromocytoma stimulation.
Gastrointestinal System: Nausea and vomiting, constipation, abdominal distension, abdominal pain, acute pancreatitis, dry mouth, dyspepsia, epigastric distress, loss of appetite, diarrhea, gastro-esophageal reflux, gastrointestinal hypomotility.
Genitourinary System: Ureteral spasm, urinary retention, dysuria, urinary frequency, urinary hesitancy, irritative bladder symptom.
Nervous System: Blurred vision, diplopia, visual disturbances, confusion, dizziness, depression, drowsiness, sedation, headache, euphoria, facial dyskinesia, false sense of well-being, feeling faint, lightheadedness, general feeling of discomfort or illness, excitability nervousness, agitation, restlessness, somnolence, insomnia, dyskinesia, irritability, tremor.
Respiratory: Dryness of the pharynx and respiratory passages, laryngismus, atelectasis, wheezing, troubled breathing, respiratory depression, hiccups.
Special Senses: labyrinthitis, tinnitus, vertigo, hypermetropia, lacrimation increased, mydriasis, photophobia.
-
7 DRUG INTERACTIONS
7.1 Benzodiazepines, Opioids, Antihistamines, Antipsychotics, Anti-anxiety Agents, or Other CNS Depressants (Including Alcohol)
The use of benzodiazepines, opioids, antihistamines, antipsychotics, anti-anxiety agents, or other CNS depressants (including alcohol) concomitantly with TUXARIN ER may cause an additive CNS depressant effect, profound sedation, respiratory depression, coma, and death and should be avoided. [see Warnings and Precautions (5.2)].
7.2 Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
Do not prescribe TUXARIN ER if the patient is taking a monoamine oxidase inhibitor (MAOI) (i.e., certain drugs used for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping a MAOI drug. The use of MAOIs or tricyclic antidepressants with codeine preparations may increase the effect of either the antidepressant or codeine.
7.3 Anticholinergic Drugs
Codeine and chlorpheniramine should be administered cautiously to persons receiving other anticholinergic drugs in order to avoid paralytic ileus and excessive anticholinergic effects.
Additive adverse effects resulting from cholinergic blockade (e.g., xerostomia, blurred vision, or constipation) may occur when anticholinergic drugs are administered with chlorpheniramine.
7.4 Inhibitors or Inducers of Metabolic Enzymes
Codeine is metabolized by the CYP2D6 and CYP3A4 isoenzymes [see Pharmacokinetics (12.3)]. The concurrent use of drugs that preferentially induce codeine N-demethylation (via CYP3A4) may increase the plasma concentrations of codeine's inactive metabolite norcodeine. Drugs that inhibit codeine O-demethylation (via CYP2D6), may decrease the plasma concentration of codeine's active metabolites, morphine and morphine-6-glucuronide. The contribution of these active metabolites to the overall antitussive effect of codeine is not known, but should be considered.
Adverse event reports in the literature suggest a possible drug interaction involving increased serum phenytoin levels and phenytoin toxicity when chlorpheniramine and phenytoin are co-administered. The exact mechanism for this interaction is not known, however it is believed that chlorpheniramine may inhibit the hepatic metabolism of phenytoin. Patients should be monitored for evidence of phenytoin toxicity such as ataxia, hyperreflexia, nystagmus and tremor when these two drugs are co-administered.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C
There are no adequate and well-controlled studies of TUXARIN ER in pregnant women.
Reproductive toxicity studies have not been conducted with TUXARIN ER; however, studies are available with individual active ingredients or related active ingredients. Because animal reproduction studies are not always predictive of human response, TUXARIN ER should be used during pregnancy only if the benefit justifies the potential risk to the fetus.
Codeine:
Codeine has embryolethal and fetotoxic effects in rats. In a study in which pregnant rats were dosed throughout organogenesis, a dose approximately 15 times the maximum recommended human daily dose (MRHDD; on a mg/m2 basis at an oral maternal dose of 120 mg/kg/day) increased resorptions and decreased fetal weight; however, these effects occurred in the presence of maternal toxicity.
In studies in which rabbits and mice were dosed throughout organogenesis, codeine at doses approximately 7 and 35 times the MRHDD (on a mg/m2 basis at 30 and 600 mg/kg/day, respectively) produced no adverse developmental effects.
Chlorpheniramine:
A retrospective study found a small, but statistically significant, association between maternal use of chlorpheniramine and inguinal hernia and eye or ear anomalies in children. Other retrospective studies have found that the frequency of congenital anomalies, in general, was not increased among offspring of women who took chlorpheniramine during pregnancy. The significance of these findings to the therapeutic use of chlorpheniramine in human pregnancy is not known.
In studies with chlorpheniramine in which pregnant rats and rabbits were dosed throughout organogenesis, oral doses up to approximately 25 and 30 times the MRHDD on a mg/m2 basis, respectively, produced no adverse developmental effects. However, when mice were dosed throughout pregnancy, a dose approximately 9 times the MRHDD (on a mg/m2 basis at an oral maternal dose of 20 mg/kg/day) was embryolethal, and postnatal survival was decreased when dosing was continued after parturition. Embryolethality was also observed when male and female rats were dosed with approximately 9 times the MRHDD (on a mg/m2 basis at an oral parental dose of 10 mg/kg/day) prior to mating.
Nonteratogenic Effects
Codeine:
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose.
8.2 Labor and Delivery
As with all opioids, administration of TUXARIN ER to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
8.3 Nursing Mothers
Risk Summary
Codeine and its active metabolite, morphine, are present in human milk. There are published studies and cases that have reported excessive sedation, respiratory depression, and death in infants exposed to codeine via breast milk. Women who are ultra-rapid metabolizers of codeine achieve higher than expected serum levels of morphine, potentially leading to higher levels of morphine in breast milk that can be dangerous in their breastfed infants. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose- dependent. There is no information on the effects of the codeine on milk production. Because of the potential for serious adverse reactions, including excess sedation, respiratory depression, and death in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with TUXARIN ER [see Warnings and Precautions (5.1)].
Clinical Considerations
If infants are exposed to TUXARIN ER through breast milk, they should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Chlorpheniramine is excreted in human milk. The clinical significance is unknown; however, the anticholinergic action of chlorpheniramine may suppress lactation if taken prior to nursing.
8.4 Pediatric Use
Safety and effectiveness of TUXARIN ER in patients under 18 years of age have not been established.
Life-threatening respiratory depression and death have occurred in children who received codeine [see Warnings and Precautions (5.1)] . In most of the reported cases, these events followed tonsillectomy and/or adenoidectomy, and many of the children had evidence of being ultra-rapid metabolizers of codeine (i.e., multiple copies of the gene for cytochrome P450 isoenzyme 2D6 or high morphine concentrations). Children with sleep apnea may be particularly sensitive to the respiratory depressant effects of codeine. Because of the risk of life-threatening respiratory depression and death:
- TUXARIN ER is contraindicated in all children younger than 12 years of age [see Contraindications (4)].
- TUXARIN ER is contraindicated for post-operative management in pediatric patients younger than 18 years of age following tonsillectomy and/or adenoidectomy [see Contraindications (4)].
- Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine. Risk factors include conditions associated with hypoventilation, such as postoperative status, obstructive sleep apnea, obesity, severe pulmonary disease, neuromuscular disease, and concomitant use of other medications that cause respiratory depression. [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical efficacy and safety studies have not been conducted with TUXARIN ER. Other reported clinical experience with the individual active ingredients of TUXARIN ER did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be made with caution, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Pharmacokinetics of TUXARIN ER has not been characterized in renal impairment subjects.
Both codeine phosphate and chlorpheniramine maleate are cleared substantially by the kidney. As such, impaired renal function could potentially lead to the risk of decreased clearance and thereby increased retention or systemic levels of both these drugs. TUXARIN ER should be used with caution in patients with severe renal impairment.
8.7 Hepatic Impairment
Pharmacokinetics of TUXARIN ER has not been characterized in hepatic impairment subjects. Both codeine and chlorpheniramine maleate are extensively metabolized by liver before elimination from the body. As such, impaired hepatic function could potentially lead to the risk of decreased metabolism and thereby increased systemic levels of both these drugs. TUXARIN ER should be used with caution in patients with severe hepatic impairment.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
TUXARIN ER is a Schedule III controlled prescription product containing codeine and should be prescribed and administered with caution.
9.2 Abuse
Codeine can produce drug dependence of the morphine type and therefore, has the potential for being abused. Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of TUXARIN ER, and it should be prescribed and administered with the same degree of caution appropriate to the use of other opioid drugs.
9.3 Dependence
Psychological dependence, physical dependence, and tolerance may develop upon repeated administration of opioids; therefore, TUXARIN ER should be prescribed and administered with caution.
Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, assumes clinically significant proportions only after several weeks of continued oral opioid use, although some mild degree of physical dependence may develop after a few days of opioid therapy.
-
10 OVERDOSAGE
No human overdosage data are available for TUXARIN ER.
Codeine
Overdosage with codeine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest, and death may occur.
Codeine may cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations.
Chlorpheniramine
Manifestations of chlorpheniramine overdosage may vary from central nervous system depression to stimulation. Central toxic effects are characterized by agitation, anxiety, delirium, disorientation, hallucinations, hyperactivity, sedation, and seizures. Severe overdosage may produce coma, medullary paralysis, and death. Peripheral toxicity includes hypertension, tachycardia, dysrhythmias, vasodilation, hyperpyrexia, mydriasis, urinary retention, and diminished gastrointestinal motility. Dry mouth, pharynx, bronchi, and nasal passages may be observed.
Impaired secretion from sweat glands following toxic doses of drugs with anticholinergic side effects may predispose to hyperthermia.
An adult ingested 400 mg chlorpheniramine with no reported serious adverse effects. Toxic psychosis, a possible class effect from overdose of sedating antihistamines, has been reported with accidental overdose of chlorpheniramine.
Treatment of overdosage consists of discontinuation of TUXARIN ER together with institution of appropriate therapy.
Give primary attention to re-establishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The opioid antagonist naloxone hydrochloride is a specific antidote for respiratory depression that may result from overdosage or unusual sensitivity to opioids including codeine. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation. For further information, see full prescribing information for naloxone hydrochloride. An antagonist should not be administered in the absence of clinically significant respiratory or circulatory depression. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Gastric emptying may be useful in removing unabsorbed drug.
Hemodialysis is not routinely used to enhance the elimination of codeine or chlorpheniramine from the body. Urinary excretion of chlorpheniramine is increased when the pH of the urine is acidic; however, acid diuresis is NOT recommended to enhance elimination in overdose, as the risks of acidemia and acute tubular necrosis in patients with rhabdomyolysis far outweigh any potential benefits.
-
11 DESCRIPTION
TUXARIN ER are extended release tablets that contain 54.3 mg of codeine phosphate (equivalent to 40 mg of codeine) and 8 mg of chlorpheniramine maleate (equivalent to 5.6 mg of chlorpheniramine).
Codeine phosphate [morphine3methyl ether phosphate (1:1) (salt)] hemihydrate, is a narcotic analgesic and antitussive. It has the following structural formula:
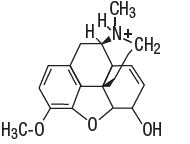
H3PO4 ∙ 1/2 H2O Codeine Phosphate Hemihydrate
C18H21NO3 ∙ H3PO4 ∙ ½H2O
MW 406.37Chlorpheniramine maleate is 2-pyridinepropanamine, γ-(4-chlorophenyl)-N,N-dimethyl-, (Z)-2-butenedioate (1:1) and has the following chemical structure:
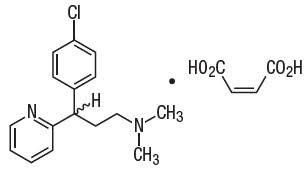
Chlorpheniramine Maleate
C16H19ClN2 ∙ C4H4O4
Molecular weight = 390.86TUXARIN ER are white to off-white, uncoated, standard round extended release matrix tablets.
Other ingredients: hypromellose, lactose monohydrate, cellulose microcrystalline, polysorbate 80, magnesium stearate, and colloidal silicon dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Codeine: Codeine is a semisynthetic narcotic antitussive and analgesic with multiple actions qualitatively similar to those of morphine. The precise mechanism of action of codeine and other opiates is not known; however, codeine is believed to act centrally on the cough center. In excessive doses, codeine will depress respiration. Codeine can produce miosis, euphoria, and physical and physiological dependence.
12.3 Pharmacokinetics
Absorption
Pharmacokinetic (PK) parameters (Mean ± SD) for TUXARIN ER in fasting, healthy volunteers are shown in the table below.
PK Parameter Single-dose Multiple-dose (BID for 6.5 days) Codeine
Mean (±SD)Chlorpheniramine Maleate
Mean (± SD)Codeine
Mean (± SD)Chlorpheniramine Maleate
Mean (± SD)Tmax (h) (Range) 3 (2-12) 6 (4-12) 3 (2-5) 5 (3-7) Cmax (ng/mL) 46 (11) 9 (3) AUCinf (ng.h/mL) for single-dose OR AUC12 (ng.h/mL) for multiple-dose 383 (99) 312 (137) Half life (h) 4 (1) 21 (7) Not determined Not determined Food Effect
The presence of a high-fat, high-calorie meal did not significantly impact the PK parameters of TUXARIN ER.
Distribution
Codeine has been reported to have an apparent volume of distribution of approximately 3-6 L/kg, indicating extensive distribution of the drug into tissues. About 7-25% of codeine, reportedly, is bound to plasma proteins. Codeine passes the blood brain barrier and the placental barrier. Small amounts of codeine and its metabolite, morphine, are transferred to human breast milk.
Chlorpheniramine is widely distributed throughout the tissues of the body, including the central nervous system. It reportedly has an apparent steady-state volume of distribution of approximately 3.2 L/kg in adults and children and is about 70% bound to plasma proteins. Chlorpheniramine and its metabolites likely cross the placental barrier and are excreted into human breast milk.
Metabolism
About 70-80% of the administered dose of codeine is metabolized by conjugation with glucuronic acid to codeine-6-glucuronide (C6G) and via O-demethylation to morphine (about 5-10%) and N-demethylation to norcodeine (about 10%) respectively. UDP-glucuronosyltransferase (UGT) 2B7 and 2B4 are the major enzymes mediating glucurodination of codeine to C6G. Cytochrome P-450 (CYP) 2D6 and CYP3A4 are the major enzymes mediating O-demethylation and N-demethylation of codeine respectively. Morphine and norcodeine are further metabolized by conjugation with glucuronic acid. Morphine and its M6 glucuronide conjugate are pharmacologically active. Whether C6G has pharmacological activity is unknown. Norcodeine and M3 glucuronide conjugate of morphine are generally not considered to be pharmacologically active.
Chlorpheniramine is rapidly and extensively metabolized via demethylation in the liver, forming mono- and didesmethyl derivatives. Oxidative metabolism of chlorpheniramine is catalyzed by cytochrome P-450 2D6.
Elimination
Approximately 90% of the total dose of codeine is excreted through the kidneys, of which approximately 10% is unchanged codeine. Plasma half-life of codeine was observed to be about 4 hours with TUXARIN ER.
Chlorpheniramine and its metabolites are primarily excreted through the kidneys, with large individual variation. Urinary excretion depends on urine pH and flow rate. Plasma half-life of chlorpheniramine was observed to be about 21 hours with TUXARIN ER.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and reproductive studies have not been conducted with TUXARIN ER however, published information is available for the active ingredients
Codeine: In 2-year studies in F344/N rats and B6C3F1 mice, codeine showed no evidence of tumorigenicity at dietary doses up to 70 and 400 mg/kg/day, respectively (approximately 9 and 25 times, respectively, the MRHDD dose for adults and children on a mg/m2 basis).
Codeine was not mutagenic in the in vitro bacterial reverse mutation assay or clastogenic in the in vitro Chinese hamster ovary (CHO) cell chromosomal aberration assay.
Fertility studies with codeine have not been conducted.
Chlorpheniramine: In 2-year studies in F344/N rats and B6C3F1 mice, chlorpheniramine maleate showed no evidence of tumorigenicity when administered 5 days/week at oral doses up to 30 and 50 mg/kg/day, respectively (approximately 25 and 20 times, respectively, the MRHDD on a mg/m2 basis).
Chlorpheniramine maleate was not mutagenic in the in vitro bacterial reverse mutation assay or the in vitro mouse lymphoma forward mutation assay. Chlorpheniramine maleate was clastogenic in the in vitro CHO cell chromosomal aberration assay.
Chlorpheniramine maleate had no effects on fertility in rats and rabbits at oral doses approximately 25 and 30 times, respectively, the MRHDD on a mg/m2 basis.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TUXARIN ER is supplied as white to off-white, uncoated, standard round tablet, debossed with MP on one side and CC on the other side. Supplied in bottles of 100 tablets: NDC: 71269-040-10.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-threatening Respiratory Depression in Children: Advise patients of the risks of respiratory depression and death with TUXARIN ER in children younger than 18 years of age. Advise patients that TUXARIN ER should not be used in children younger than 12 years of age or in a child younger than 18 years of age for treatment after tonsillectomy and/or adenoidectomy [see Warnings and Precautions (5.1)] .
Overdosage: Advise patients not to increase the dose or dosing frequency of TUXARIN ER because serious adverse events such as respiratory depression may occur with overdosage. [see Warnings and Precautions (5.2); Overdosage (10)]
Interactions with Benzodiazepines and Other Central Nervous System Depressants: Inform patients and caregivers that potentially fatal additive effects may occur if TUXARIN ER is used with benzodiazepines or other CNS depressants, including alcohol. Because of this risk, patients should avoid concomitant use of TUXARIN ER with benzodiazepines or other CNS depressants, including alcohol [see Warnings and Precautions (5.3), Drug Interactions (7.1)].
Activities Requiring Mental Alertness: Caution patients that TUXARIN ER may produce marked drowsiness and impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. [see Warnings and Precautions (5.6)]
Controlled Substance Status/Potential for Abuse and Dependence: Caution patients that TUXARIN ER contains codeine and can produce drug dependence. [see Abuse and Dependence (9.2, 9.3)].
Lactation: Advise women that breastfeeding is not recommended during treatment with TUXARIN ER [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
TUXARIN ER™ (tuks a ren)
(codeine phosphate and chlorpheniramine maleate) extended release tablets, CIIIThis Medication Guide has been approved by the U.S. Food and Drug Administration Issued: August 2017 What is the most important information I should know about TUXARIN ER?
- TUXARIN ER can cause children to stop breathing (respiratory depression) and cause death.
- Do not give TUXARIN ER to children younger than 12 years of age, or to children younger than 18 years of age after tonsillectomy or adenoidectomy surgery.
- You should not give TUXARIN ER to children between 12 to 18 years of age who have certain risk factors for breathing problems, including:
- recent surgery
- obstructive sleep apnea
- are obese
- severe lung problems
- other medical conditions that can cause difficulty breathing, such as neuromuscular problems
- taking other medicines that cause breathing problems
- You should not breastfeed during treatment with TUXARIN ER because it may cause your child to stop breathing and cause death.
- Severe drowsiness, breathing problems (respiratory depression), coma, and death can happen in adults and children who take TUXARIN ER with benzodiazepines, or other central nervous system depressants, including alcohol.
- Avoid taking other medicines that can cause drowsiness or sleepiness, or drinking alcohol during treatment with TUXARIN ER. Ask your healthcare provider for a list of these medicines if you are not sure.
- TUXARIN ER can cause breathing problems (respiratory depression) and drowsiness.
- Take TUXARIN ER exactly as prescribed by your healthcare provider. If you take the wrong dose of TUXARIN ER, you could overdose and die. See the Medication Guide section called "How should I take TUXARIN ER?" for important information about how to take TUXARIN ER correctly.
- Avoid driving a car or operating machinery during treatment with TUXARIN ER.
- Call your healthcare provider or get emergency medical help right away if anyone taking TUXARIN ER, or your breastfeeding baby has any of the symptoms listed below:
- increased sleepiness
- difficulty breathing
- limpness
- confusion
- shallow breathing
- your baby has difficulty breastfeeding
Keep TUXARIN ER in a safe place away from children. Accidental use by a child is a medical emergency and can cause death. If a child accidentally takes TUXARIN ER, get emergency help right away. What is TUXARIN ER? - TUXARIN ER is a prescription medicine used to treat cough and upper respiratory symptoms that you can have with allergies or a common cold. TUXARIN ER is for adults 18 years and older. TUXARIN ER contains 2 medicines, codeine and chlorpheniramine. Codeine is a narcotic cough suppressant. Chlorpheniramine is an antihistamine.
- TUXARIN ER is a federal controlled substance (CIII) because it contains codeine that can be abused or lead to dependence. Keep TUXARIN ER in a safe place to prevent misuse and abuse. Selling or giving away TUXARIN ER may harm others and is against the law.
- TUXARIN ER is not for children under 18 years of age. It is not known if TUXARIN ER is safe and effective in children.
Who should not take TUXARIN ER? - Do not give TUXARIN ER to any children younger than 12 years of age.
- Do not give TUXARIN ER to children younger than 18 years of age following tonsillectomy or adenoidectomy surgery.
- Do not take TUXARIN ER if you are allergic to any of the ingredients in TUXARIN ER. See the end of this Medication Guide for a complete list of ingredients in TUXARIN ER. You may have an increased risk of having an allergic reaction to TUXARIN ER if you are allergic to certain other opioid medicines.
Before taking TUXARIN ER, tell your healthcare provider about all of your medical conditions, including if you: - have a drug dependence
- have lung or breathing problems
- have had a head injury
- have pain in your stomach (abdomen)
- have constipation or problems with your intestines
- have prostate problems
- have problems with your urinary tract (urethral stricture)
- plan to have surgery
- abuse alcohol
- have kidney or liver problems
- have thyroid problems, such as hypothyroidism
- have Addison's disease
- are pregnant or plan to become pregnant. It is not known if TUXARIN ER will harm your unborn baby. You and your healthcare provider should decide if you should take TUXARIN ER while you are pregnant.
- are breastfeeding or plan to breastfeed. Codeine and chlorpheniramine pass into your breast milk and may harm your baby. You and your healthcare provider should discuss whether you should take TUXARIN ER or breastfeed. You should not do both.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using TUXARIN ER with certain other medicines may affect each other. Using TUXARIN ER with other medicines can cause serious side effects. Especially tell your healthcare provider if you: - take pain medicines such as narcotics
- take cold or allergy medicines that contain antihistamines or cough suppressants
- take medicines for mental illness (anti-psychotics, anti-anxiety)
- drink alcohol
- take medicines for depression, other mental health problems, or Parkinson's disease, including monoamine oxidase inhibitors (MAOIs), or if you have taken an MAOI in the last 14 days
- take medicines for stomach or intestine problems
Ask your healthcare provider if you are not sure if you take one of these medicines.
- Take TUXARIN ER exactly as your healthcare provider tells you.
- Do not take more than 2 TUXARIN ER tablets in 24 hours.
- If you take too much TUXARIN ER, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking TUXARIN ER? - TUXARIN ER can cause you to be drowsy. Avoid driving a car or using machinery during treatment with TUXARIN ER.
- Do not drink alcohol during treatment with TUXARIN ER. Drinking alcohol can increase your chances of having serious side effects.
What are the possible side effects of TUXARIN ER?
TUXARIN ER may cause serious side effects, including:- See "What is the most important information I should know about TUXARIN ER"
- Breathing problems (respiratory depression) which can lead to death. Call your healthcare provider or get emergency treatment right away if you have excessive sleepiness, shallow or slow breathing, or confusion.
- Physical dependence or abuse. Take TUXARIN ER exactly as your healthcare provider tells you to take it.
Stopping TUXARIN ER suddenly could cause withdrawal symptoms. - Increased intracranial pressure
- Bowel problems including constipation or stomach pain.
The most common side effects of TUXARIN ER include: - nausea and vomiting
- constipation
- swelling or bloating of your stomach-area
- stomach-area pain
- vision problems, including blurred vision and double vision
- confusion
- dizziness
- depression
- drowsiness
- headache
- feeling high (euphoria)
- feeling faint
- light-headedness
- excitability, nervousness
- agitation, restlessness, irritability
- sleepiness
- difficulty sleeping (insomnia)
- unable to control muscle movements
- tremor
- general feeling of discomfort or illness
These are not all the possible side effects of TUXARIN ER. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store TUXARIN ER? - Store TUXARIN ER in a safe place between 68°F to 77°F (20°C to 25°C).
- Keep TUXARIN ER in a tightly closed, child-resistant container and out of the light.
- Safely throw away medicine that is out of date or no longer needed.
- Keep TUXARIN ER and all medicines out of the reach of children.
General information about the safe and effective use of TUXARIN ER. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TUXARIN ER for a condition for which it was not prescribed. Do not give TUXARIN ER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TUXARIN ER that is written for health professionals. What are the ingredients in TUXARIN ER? Active ingredients: codeine and chlorpheniramine Inactive ingredients: Hypromellose, lactose monohydrate, cellulose microcrystalline, polysorbate 80, magnesium stearate, and colloidal silicon dioxide. Distributed By: MainPointe Pharmaceuticals, LLC
Louisville, KY, 40202For more information go to mainpointepharmaceuticals.com or call 502-709-7544 - TUXARIN ER can cause children to stop breathing (respiratory depression) and cause death.
-
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
NDC: 71269-040-10
CIII
Tuxarin ER™
(codeine phosphate and
chlorpheniramine maleate
extended-release tablets)
54.3 mg/ 8 mgEach Tablet Contains:
Codeine Phosphate, USP 54.3 mg
(Equivalent to 40 mg of Codeine)
Chlorpheniramine Maleate, USP 8 mg
(Equivalent to 5.6 mg of Chlorpheniramine)PHARMACIST: PLEASE DISPENSE WITH
MEDICATION GUIDERx only
100 Tablets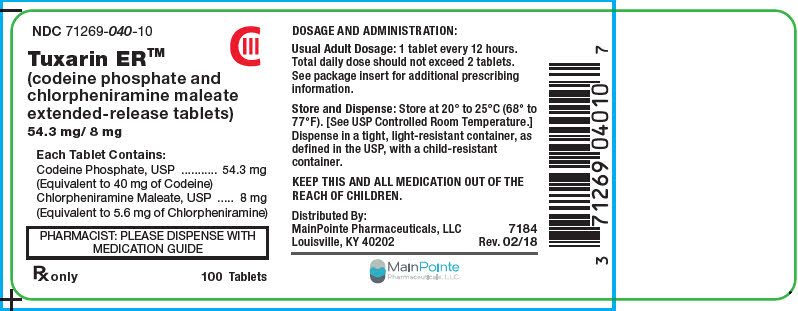
-
INGREDIENTS AND APPEARANCE
TUXARIN
codeine phosphate and chlorpheniramine maleate tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71269-040 Route of Administration ORAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 54.3 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 8 mg Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code MP;CC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71269-040-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206323 10/15/2018 Labeler - Mainpointe Pharmaceuticals (080544378) Establishment Name Address ID/FEI Business Operations NexGen Pharma 160356114 MANUFACTURE(71269-040)
Trademark Results [Tuxarin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TUXARIN 97027082 not registered Live/Pending |
MainPointe Pharmaceuticals, LLC 2021-09-14 |
 TUXARIN 86659512 not registered Dead/Abandoned |
Spriaso LLC 2015-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.