Rainbow Instant Hand Sanitizer (gel - 1.5 oz.) NDC: 69060-0002-1 Rainbow Instant Hand Sanitizer (gel - 8 oz.) NDC: 69060-0002-8
Rainbow Instant Hand Sanitizer by
Drug Labeling and Warnings
Rainbow Instant Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Rainbow Technology Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RAINBOW INSTANT HAND SANITIZER- alcohol gel
Rainbow Technology Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rainbow Instant Hand Sanitizer (gel - 1.5 oz.)
NDC: 69060-0002-1
Rainbow Instant Hand Sanitizer (gel - 8 oz.)
NDC: 69060-0002-8
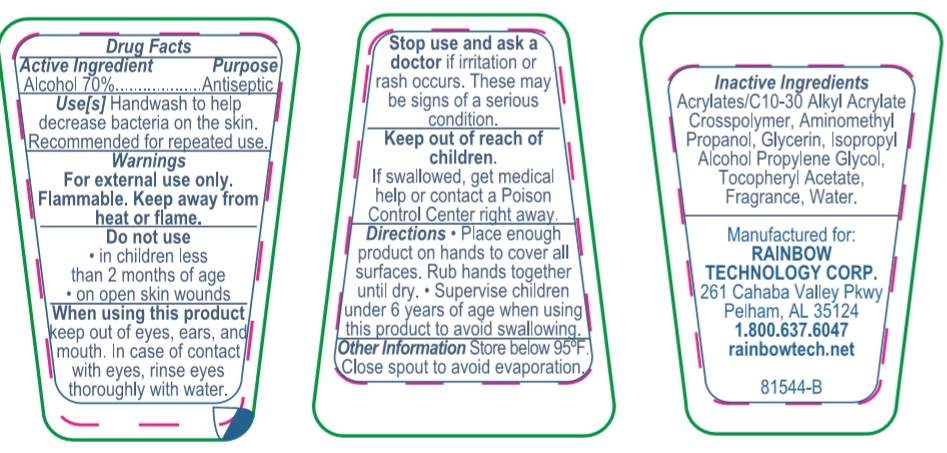
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Inactive ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Glycerin, Isopropyl Alcohol, Propylene Glycol, Tocopheryl Acetate, Fragrance, Water.
Package Label - Principal Display Panel
RAINBOW TECHNOLOGY CORP.
261 Cahaba Valley Parkway
Pelham, Alabama 35124-1146
Rainbow Instant Hand Sanitizer (1.5 oz./ 44 mL)
NDC: 69060-0002-1

Rainbow Instant Hand Sanitizer (8 oz./237 mL)
NDC: 69060-0002-8

| RAINBOW INSTANT HAND SANITIZER
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rainbow Technology Corporation (055905624) |