SKINSEI by Natures Formulae Health Products Ltd. Skinsei Hand Sanitizer
SKINSEI by
Drug Labeling and Warnings
SKINSEI by is a Otc medication manufactured, distributed, or labeled by Natures Formulae Health Products Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SKINSEI- ethyl alcohol liquid liquid
Natures Formulae Health Products Ltd.
----------
Skinsei Hand Sanitizer
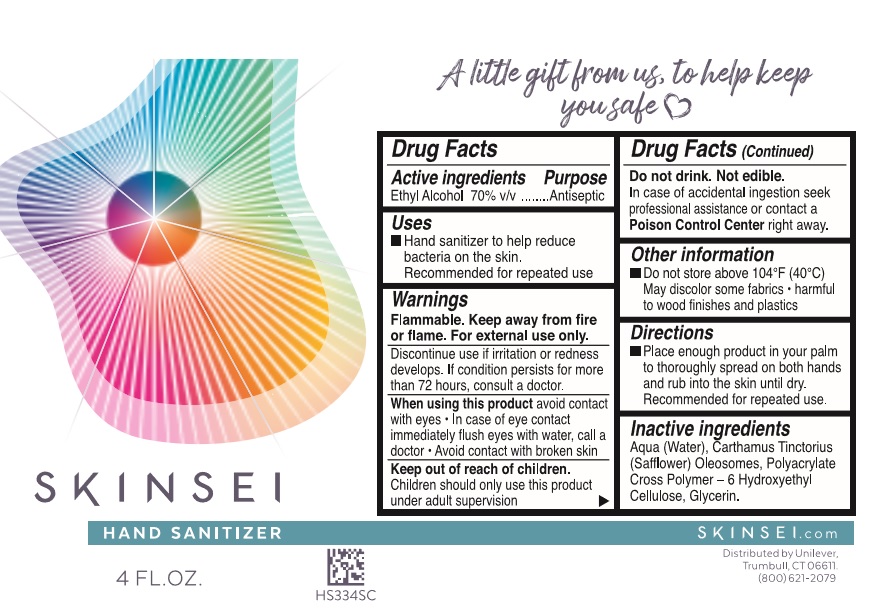
Warnings
Flammable. Keep away from fire or flame
For external use only
Discontinue use if irritation or redness develops. If condition persists for more than 72 hours, consult a doctor.
When using this productavoid contact with eyes · In case of contact, flush eyes with water, call a doctor · Avoid contact with broken skin
Do not drink. Not edible.
In case of accidental ingeston seek professional assistance or contact a Poison Contro Center right away.
Directions
Place enough product in your palm to thoroughly spread on both hands and rub into the skin until dry. Recommended for repeated use.
Other information
Do not store above 104˚F (40˚C). May discolor some fabrics · harmful to wood finishes and plastics
| SKINSEI
ethyl alcohol liquid liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Natures Formulae Health Products Ltd. (241385587) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Natures Formulae Health Products Ltd. | 241385587 | manufacture(69204-024) | |
Trademark Results [SKINSEI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SKINSEI 87444658 not registered Live/Pending |
Conopco, Inc. 2017-05-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.