These highlights do not include all the information needed to use WEZLANA™ safely and effectively. See full prescribing information for WEZLANA. WEZLANA™ (ustekinumab-auub) injection, for subcutaneous or intravenous use Initial U.S. Approval: 2023 WEZLANA™ (ustekinumab-auub) is biosimilarBiosimilar means that the biological product is approved based on data demonstrating that it is highly similar to an FDA-approved biological product known as a reference product, and that there are no clinically meaningful differences between the biosimilar product and the reference product. Biosimilarity of WEZLANA has been demonstrated for the condition(s) of use (e.g., indication(s), dosing regimen(s), strength(s), dosage form(s), and route(s) of administration described in its Full Prescribing Information. to STELARA® (ustekinumab).
WEZLANA by
Drug Labeling and Warnings
WEZLANA by is a Prescription medication manufactured, distributed, or labeled by Amgen USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WEZLANA- ustekinumab-auub injection, solution
Amgen USA Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use WEZLANA™ safely and effectively. See full prescribing information for WEZLANA.
WEZLANA™ (ustekinumab-auub) injection, for subcutaneous or intravenous use Initial U.S. Approval: 2023 WEZLANA™ (ustekinumab-auub) is biosimilar* to STELARA® (ustekinumab). INDICATIONS AND USAGEWEZLANA is a human interleukin -12 and -23 antagonist indicated for the treatment of: Adult patients with:
Pediatric patients 6 years and older with: DOSAGE AND ADMINISTRATIONPsoriasis Adult Subcutaneous Recommended Dosage (2.1):
Psoriasis Pediatric Patients (6 to 17 years old) Subcutaneous Recommended Dosage (2.1): Weight-based dosing is recommended at the initial dose, 4 weeks later, then every 12 weeks thereafter.
Psoriatic Arthritis Adult Subcutaneous Recommended Dosage (2.2):
Psoriatic Arthritis Pediatric (6 to 17 years old) Subcutaneous Recommended Dosage (2.2): Weight-based dosing is recommended at the initial dose, 4 weeks later, then every 12 weeks thereafter.
Crohn's Disease and Ulcerative Colitis Initial Adult Intravenous Recommended Dosage (2.3): A single intravenous infusion using weight-based dosing:
Crohn's Disease and Ulcerative Colitis Maintenance Adult Subcutaneous Recommended Dosage (2.3): A subcutaneous 90 mg dose 8 weeks after the initial intravenous dose, then every 8 weeks thereafter. DOSAGE FORMS AND STRENGTHSSubcutaneous Injection (3)
Intravenous Infusion (3)
CONTRAINDICATIONSClinically significant hypersensitivity to ustekinumab products or to any of the excipients in WEZLANA. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions are:
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 12/2024 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis (PsO)
WEZLANA is indicated for the treatment of adults and pediatric patients 6 years of age and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
1.2 Psoriatic Arthritis (PsA)
WEZLANA is indicated for the treatment of adults and pediatric patients 6 years of age and older with active psoriatic arthritis.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Plaque Psoriasis
Subcutaneous Adult Dosage Regimen
- For patients weighing 100 kg or less, the recommended dosage is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients weighing more than 100 kg, the recommended dosage is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
In subjects weighing more than 100 kg, 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects [see Clinical Studies (14)].
Subcutaneous Pediatric Dosage Regimen
Administer WEZLANA subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of WEZLANA for pediatric patients (6–17 years old) with plaque psoriasis based on body weight is shown below (Table 1).
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
| less than 60 kg | 0.75 mg/kg |
| 60 kg to 100 kg | 45 mg |
| more than 100 kg | 90 mg |
For pediatric patients weighing less than 60 kg, the administration volume for the recommended dose (0.75 mg/kg) is shown in Table 2; withdraw the appropriate volume from the single-dose vial.
| Body Weight (kg) at the time of dosing | Dose (mg) | Volume of injection (mL) |
|---|---|---|
|
|
||
| 15 | 11.3 | 0.12 |
| 16 | 12.0 | 0.13 |
| 17 | 12.8 | 0.14 |
| 18 | 13.5 | 0.15 |
| 19 | 14.3 | 0.16 |
| 20 | 15.0 | 0.17 |
| 21 | 15.8 | 0.17 |
| 22 | 16.5 | 0.18 |
| 23 | 17.3 | 0.19 |
| 24 | 18.0 | 0.20 |
| 25 | 18.8 | 0.21 |
| 26 | 19.5 | 0.22 |
| 27 | 20.3 | 0.22 |
| 28 | 21.0 | 0.23 |
| 29 | 21.8 | 0.24 |
| 30 | 22.5 | 0.25 |
| 31 | 23.3 | 0.26 |
| 32 | 24.0 | 0.27 |
| 33 | 24.8 | 0.27 |
| 34 | 25.5 | 0.28 |
| 35 | 26.3 | 0.29 |
| 36 | 27.0 | 0.30 |
| 37 | 27.8 | 0.31 |
| 38 | 28.5 | 0.32 |
| 39 | 29.3 | 0.32 |
| 40 | 30.0 | 0.33 |
| 41 | 30.8 | 0.34 |
| 42 | 31.5 | 0.35 |
| 43 | 32.3 | 0.36 |
| 44 | 33.0 | 0.37 |
| 45 | 33.8 | 0.37 |
| 46 | 34.5 | 0.38 |
| 47 | 35.3 | 0.39 |
| 48 | 36.0 | 0.40 |
| 49 | 36.8 | 0.41 |
| 50 | 37.5 | 0.42 |
| 51 | 38.3 | 0.42 |
| 52 | 39.0 | 0.43 |
| 53 | 39.8 | 0.44 |
| 54 | 40.5 | 0.45 |
| 55 | 41.3 | 0.46 |
| 56 | 42.0 | 0.46 |
| 57 | 42.8 | 0.47 |
| 58 | 43.5 | 0.48 |
| 59 | 44.3 | 0.49 |
2.2 Recommended Dosage in Psoriatic Arthritis
Subcutaneous Adult Dosage Regimen
- The recommended dosage is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks.
- For patients with co-existent moderate-to-severe plaque psoriasis weighing more than 100 kg, the recommended dosage is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.
Subcutaneous Pediatric Dosage Regimen
Administer WEZLANA subcutaneously at Weeks 0 and 4, then every 12 weeks thereafter.
The recommended dose of WEZLANA for pediatric patients (6 to 17 years old) with psoriatic arthritis, based on body weight, is shown below (Table 3).
| Body Weight of Patient at the Time of Dosing | Recommended Dose |
|---|---|
|
|
|
| less than 60 kg* | 0.75 mg/kg |
| 60 kg or more | 45 mg |
| greater than 100 kg with co-existent moderate-to-severe plaque psoriasis | 90 mg |
2.3 Recommended Dosage in Crohn's Disease and Ulcerative Colitis
Intravenous Induction Adult Dosage Regimen
A single intravenous infusion dose of WEZLANA using the weight-based dosage regimen specified in Table 4 [see Instructions for dilution of WEZLANA 130 mg vial for intravenous infusion (2.6)].
| Body Weight of Patient at the time of dosing | Dose | Number of 130 mg/26 mL (5 mg/mL) WEZLANA vials |
|---|---|---|
| 55 kg or less | 260 mg | 2 |
| more than 55 kg to 85 kg | 390 mg | 3 |
| more than 85 kg | 520 mg | 4 |
2.4 General Considerations for Administration
- WEZLANA is intended for use under the guidance and supervision of a healthcare provider. WEZLANA should only be administered to patients who will be closely monitored and have regular follow-up visits with a healthcare provider. The appropriate dose should be determined by a healthcare provider using the patient's current weight at the time of dosing. In pediatric patients, it is recommended that WEZLANA be administered by a healthcare provider. If a healthcare provider determines that it is appropriate, a patient may self-inject, or a caregiver may inject WEZLANA after proper training in subcutaneous injection technique. Instruct patients to follow the directions provided in the Medication Guide [see Medication Guide].
- The needle cap on the prefilled syringe does not contain dry natural rubber (a derivative of latex). The prefilled ConfiPen autoinjector is not made with natural rubber latex.
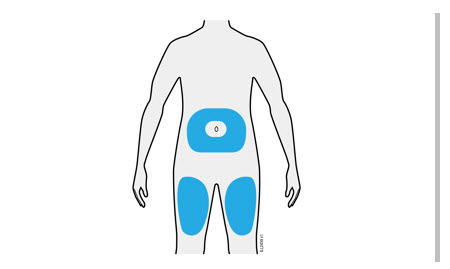
- It is recommended that each injection be administered at a different anatomic location (such as upper arms, gluteal regions, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, or indurated. When using the single-dose vial, a 1 mL syringe with a 27 gauge, ½ inch needle is recommended.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. WEZLANA is a clear to opalescent and colorless to light yellow solution. Do not use WEZLANA if it is discolored or cloudy, or if other particulate matter is present. WEZLANA does not contain preservatives; therefore, discard any unused product remaining in the ConfiPen autoinjector, vial and/or syringe.
2.5 Instructions for Administration of WEZLANA Prefilled Syringes Equipped with Needle Safety Guard
Refer to the diagram below for the provided instructions.
To prevent premature activation of the needle safety guard, do not touch the NEEDLE GUARD CLIPS at any time during use.
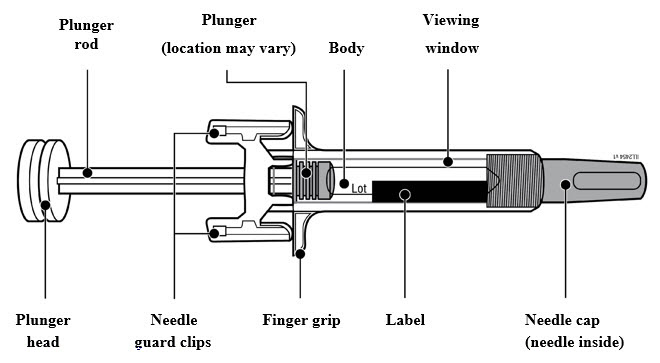
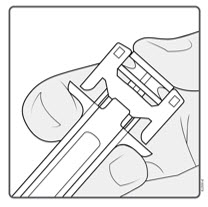
- Hold the BODY and remove the NEEDLE CAP. Do not hold the PLUNGER or PLUNGER HEAD while removing the NEEDLE CAP or the PLUNGER may move. Do not use the prefilled syringe if it is dropped without the NEEDLE CAP in place.
Inject WEZLANA subcutaneously as recommended [see Dosage and Administration (2.1, 2.2, 2.3)]. - Inject all of the medication by pushing in the PLUNGER until the PLUNGER HEAD is completely between the finger grip. Injection of the entire prefilled syringe contents is necessary to activate the needle guard.
- After injection, maintain the pressure on the PLUNGER HEAD and remove the needle from the skin. Slowly take your thumb off the PLUNGER HEAD to allow the empty syringe to move up until the entire needle is covered by the needle guard, as shown by the illustration below:
Used syringes should be placed in a puncture-resistant container.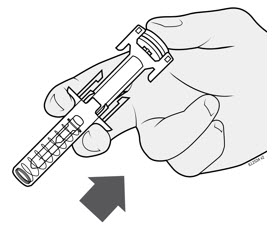
2.6 Instructions for Administration of WEZLANA Prefilled ConfiPen Autoinjector
The WEZLANA single-dose prefilled ConfiPen autoinjector "Instructions for Use" insert contains detailed instructions on preparation, injection site selection, administration and disposal.
2.7 Preparation and Administration of WEZLANA 130 mg/26 mL (5 mg/mL) Vial for Intravenous Infusion (Crohn's Disease and Ulcerative Colitis)
WEZLANA solution for intravenous infusion must be diluted, prepared and infused by a healthcare professional using aseptic technique.
- Calculate the dose and the number of WEZLANA vials needed based on patient weight (Table 4). Each 26 mL vial of WEZLANA contains 130 mg of ustekinumab-auub.
- Withdraw, and then discard a volume of the 0.9% Sodium Chloride Injection, USP from the 250 mL infusion bag equal to the volume of WEZLANA to be added (discard 26 mL sodium chloride for each vial of WEZLANA needed, for 2 vials- discard 52 mL, for 3 vials- discard 78 mL, 4 vials- discard 104 mL). Alternatively, a 250 mL infusion bag containing 0.45% Sodium Chloride Injection, USP may be used.
- Withdraw 26 mL of WEZLANA from each vial needed and add it to the 250 mL infusion bag. The final volume in the infusion bag should be 250 mL. Gently mix.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
- Infuse the diluted solution over a period of at least one hour. Once diluted, the infusion should be completely administered within eight hours of the dilution in the infusion bag.
- Use only an infusion set with an in-line, sterile, non-pyrogenic, low protein-binding filter (pore size 0.2 micrometer).
- Do not infuse WEZLANA concomitantly in the same intravenous line with other agents.
- WEZLANA does not contain preservatives. Each vial is for one-time use in only one patient. Discard any remaining solution. Dispose any unused medicinal product in accordance with local requirements.
Storage
If necessary, the diluted infusion solution may be kept at room temperature up to 25°C (77°F) for up to 7 hours. Storage time at room temperature begins once the diluted solution has been prepared. The infusion should be completed within 8 hours after the dilution in the infusion bag (cumulative time after preparation including the storage and the infusion period). Do not freeze. Discard any unused portion of the infusion solution.
3 DOSAGE FORMS AND STRENGTHS
WEZLANA (ustekinumab-auub) is a clear to opalescent and colorless to light yellow solution.
4 CONTRAINDICATIONS
WEZLANA is contraindicated in patients with clinically significant hypersensitivity to ustekinumab products or to any of the excipients in WEZLANA [see Warnings and Precautions (5.5)].
5 WARNINGS AND PRECAUTIONS
5.1 Infections
Ustekinumab products may increase the risk of infections and reactivation of latent infections. Serious bacterial, mycobacterial, fungal, and viral infections were observed in patients receiving ustekinumab products [see Adverse Reactions (6.1, 6.3)].
Serious infections requiring hospitalization, or otherwise clinically significant infections, reported in clinical trials included the following:
- Plaque Psoriasis: diverticulitis, cellulitis, pneumonia, appendicitis, cholecystitis, sepsis, osteomyelitis, viral infections, gastroenteritis and urinary tract infections.
- Psoriatic arthritis: cholecystitis.
- Crohn's disease: anal abscess, gastroenteritis, ophthalmic herpes zoster, pneumonia, and listeria meningitis.
- Ulcerative colitis: gastroenteritis, ophthalmic herpes zoster, pneumonia, and listeriosis.
Avoid initiating treatment with WEZLANA in patients with any clinically important active infection until the infection resolves or is adequately treated. Consider the risks and benefits of treatment prior to initiating use of WEZLANA in patients with a chronic infection or a history of recurrent infection.
Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur while on treatment with WEZLANA and discontinue WEZLANA for serious or clinically significant infections until the infection resolves or is adequately treated.
5.2 Theoretical Risk for Vulnerability to Particular Infections
Individuals genetically deficient in IL-12/IL-23 are particularly vulnerable to disseminated infections from mycobacteria (including nontuberculous, environmental mycobacteria), salmonella (including nontyphi strains), and Bacillus Calmette-Guerin (BCG) vaccinations. Serious infections and fatal outcomes have been reported in such patients.
It is not known whether patients with pharmacologic blockade of IL-12/IL-23 from treatment with ustekinumab products may be susceptible to these types of infections. Consider appropriate diagnostic testing (e.g., tissue culture, stool culture, as dictated by clinical circumstances).
5.3 Pre-treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis infection prior to initiating treatment with WEZLANA.
Avoid administering WEZLANA to patients with active tuberculosis infection. Initiate treatment of latent tuberculosis prior to administering WEZLANA. Consider anti-tuberculosis therapy prior to initiation of WEZLANA in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed. Closely monitor patients receiving WEZLANA for signs and symptoms of active tuberculosis during and after treatment.
5.4 Malignancies
Ustekinumab products are immunosuppressants and may increase the risk of malignancy. Malignancies were reported among subjects who received ustekinumab in clinical trials [see Adverse Reactions (6.1)]. In rodent models, inhibition of IL-12/IL-23p40 increased the risk of malignancy [see Nonclinical Toxicology (13)].
The safety of ustekinumab products has not been evaluated in patients who have a history of malignancy or who have a known malignancy.
There have been postmarketing reports of the rapid appearance of multiple cutaneous squamous cell carcinomas in patients receiving ustekinumab products who had pre-existing risk factors for developing non-melanoma skin cancer. Monitor all patients receiving WEZLANA for the appearance of non-melanoma skin cancer. Closely follow patients greater than 60 years of age, those with a medical history of prolonged immunosuppressant therapy and those with a history of PUVA treatment [see Adverse Reactions (6.1)].
5.5 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with ustekinumab products [see Adverse Reactions (6.1, 6.3)]. If an anaphylactic or other clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue WEZLANA.
5.6 Posterior Reversible Encephalopathy Syndrome (PRES)
Two cases of posterior reversible encephalopathy syndrome (PRES), also known as Reversible Posterior Leukoencephalopathy Syndrome (RPLS), were reported in clinical trials. Cases have also been reported in postmarketing experience in patients with psoriasis, psoriatic arthritis and Crohn's disease. Clinical presentation included headaches, seizures, confusion, visual disturbances, and imaging changes consistent with PRES a few days to several months after ustekinumab product initiation. A few cases reported latency of a year or longer. Patients recovered with supportive care following withdrawal of ustekinumab products.
Monitor all patients treated with WEZLANA for signs and symptoms of PRES. If PRES is suspected, promptly administer appropriate treatment, and discontinue WEZLANA.
5.7 Immunizations
Prior to initiating therapy with WEZLANA, patients should receive all age-appropriate immunizations as recommended by current immunization guidelines. Patients being treated with WEZLANA should avoid receiving live vaccines. Avoid administering BCG vaccines during treatment with WEZLANA or for one year prior to initiating treatment or one year following discontinuation of treatment. Caution is advised when administering live vaccines to household contacts of patients receiving WEZLANA because of the potential risk for shedding from the household contact and transmission to patient.
Non-live vaccinations received during a course of WEZLANA may not elicit an immune response sufficient to prevent disease.
5.8 Noninfectious Pneumonia
Cases of interstitial pneumonia, eosinophilic pneumonia and cryptogenic organizing pneumonia have been reported during post-approval use of ustekinumab products. Clinical presentations included cough, dyspnea, and interstitial infiltrates following one to three doses. Serious outcomes have included respiratory failure and prolonged hospitalization. Patients improved with discontinuation of therapy and in certain cases administration of corticosteroids. If diagnosis is confirmed, discontinue WEZLANA and institute appropriate treatment [see Postmarketing Experience (6.3)].
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the label:
- Infections [see Warnings and Precautions (5.1)]
- Malignancies [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Posterior Reversible Encephalopathy Syndrome (PRES) [see Warnings and Precautions (5.6)]
- Noninfectious Pneumonia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Subjects with Plaque Psoriasis
The safety data reflect exposure to ustekinumab in 3117 adult subjects with plaque psoriasis, including 2414 exposed for at least 6 months, 1855 exposed for at least one year, 1653 exposed for at least two years, 1569 exposed for at least three years, 1482 exposed for at least four years and 838 exposed for at least five years.
Table 5 summarizes the adverse reactions that occurred at a rate of at least 1% with higher rates in the ustekinumab groups during the placebo-controlled period of Ps STUDY 1 and Ps STUDY 2 [see Clinical Studies (14)].
| Ustekinumab | |||
|---|---|---|---|
| Subjects treated | Placebo 665 | 45 mg 664 | 90 mg 666 |
| Nasopharyngitis | 51 (8%) | 56 (8%) | 49 (7%) |
| Upper respiratory tract infection | 30 (5%) | 36 (5%) | 28 (4%) |
| Headache | 23 (3%) | 33 (5%) | 32 (5%) |
| Fatigue | 14 (2%) | 18 (3%) | 17 (3%) |
| Back pain | 8 (1%) | 9 (1%) | 14 (2%) |
| Dizziness | 8 (1%) | 8 (1%) | 14 (2%) |
| Pharyngolaryngeal pain | 7 (1%) | 9 (1%) | 12 (2%) |
| Pruritus | 9 (1%) | 10 (2%) | 9 (1%) |
| Injection site erythema | 3 (< 1%) | 6 (1%) | 13 (2%) |
| Myalgia | 4 (1%) | 7 (1%) | 8 (1%) |
| Depression | 3 (< 1%) | 8 (1%) | 4 (1%) |
Adverse reactions that occurred at rates less than 1% in the controlled period of Ps STUDIES 1 and 2 through week 12 included: cellulitis, herpes zoster, diverticulitis and certain injection site reactions (pain, swelling, pruritus, induration, hemorrhage, bruising, and irritation).
One case of PRES occurred during adult plaque psoriasis clinical trials [see Warnings and Precautions (5.6)].
Infections
In the placebo-controlled period of clinical trials of subjects with plaque psoriasis (average follow-up of 12.6 weeks for placebo-treated subjects and 13.4 weeks for ustekinumab-treated subjects), 27% of ustekinumab-treated subjects reported infections (1.39 per subject-year of follow-up) compared with 24% of placebo-treated subjects (1.21 per subject-year of follow-up). Serious infections occurred in 0.3% of ustekinumab-treated subjects (0.01 per subject-year of follow-up) and in 0.4% of placebo-treated subjects (0.02 per subject-year of follow-up) [see Warnings and Precautions (5.1)].
In the controlled and non-controlled portions of plaque psoriasis clinical trials (median follow-up of 3.2 years), representing 8998 subject-years of exposure, 72.3% of ustekinumab-treated subjects reported infections (0.87 per subject-years of follow-up). Serious infections were reported in 2.8% of subjects (0.01 per subject-years of follow-up).
Malignancies
In the controlled and non-controlled portions of plaque psoriasis clinical trials (median follow-up of 3.2 years, representing 8998 subject-years of exposure), 1.7% of ustekinumab-treated subjects reported malignancies excluding non-melanoma skin cancers (0.60 per hundred subject-years of follow-up).
Non-melanoma skin cancer was reported in 1.5% of ustekinumab-treated subjects (0.52 per hundred subject-years of follow-up) [see Warnings and Precautions (5.4)]. The most frequently observed malignancies other than non-melanoma skin cancer during the clinical trials were: prostate, melanoma, colorectal and breast. Malignancies other than non-melanoma skin cancer in ustekinumab-treated subjects during the controlled and uncontrolled portions of trials were similar in type and number to what would be expected in the general U.S. population according to the SEER database (adjusted for age, gender and race).1
Pediatric Subjects with Plaque Psoriasis
The safety of ustekinumab was assessed in two trials of pediatric subjects with moderate to severe plaque psoriasis. Ps STUDY 3 evaluated safety for up to 60 weeks in 110 pediatric subjects 12 to 17 years old. Ps STUDY 4 evaluated safety for up to 56 weeks in 44 pediatric subjects 6 to 11years old. The safety profile in pediatric subjects was similar to the safety profile from trials in adults with plaque psoriasis.
Psoriatic Arthritis
The safety of ustekinumab was assessed in 927 subjects in two randomized, double-blind, placebo-controlled trials in adults with active psoriatic arthritis (PsA). The overall safety profile of ustekinumab in subjects with PsA was consistent with the safety profile seen in adult psoriasis clinical trials. A higher incidence of arthralgia, nausea, and dental infections was observed in ustekinumab-treated subjects when compared with placebo-treated subjects (3% vs. 1% for arthralgia and 3% vs. 1% for nausea; 1% vs. 0.6% for dental infections) in the placebo-controlled portions of the PsA clinical trials.
Crohn's Disease
The safety of ustekinumab was assessed in 1407 subjects with moderately to severely active Crohn's disease (Crohn's Disease Activity Index [CDAI] greater than or equal to 220 and less than or equal to 450) in three randomized, double-blind, placebo-controlled, parallel-group, multicenter trials. These 1407 subjects included 40 subjects who received a prior investigational intravenous ustekinumab formulation but were not included in the efficacy analyses. In trials CD-1 and CD-2 there were 470 subjects who received ustekinumab 6 mg/kg as a weight-based single intravenous induction dose and 466 who received placebo [see Dosage and Administration (2.3)]. Subjects who were responders in either trial CD-1 or CD-2 were randomized to receive a subcutaneous maintenance regimen of either 90 mg ustekinumab every 8 weeks, or placebo for 44 weeks in trial CD-3. Subjects in these 3 trials may have received other concomitant therapies including aminosalicylates, immunomodulatory agents [azathioprine (AZA), 6-mercaptopurine (6-MP), methotrexate (MTX)], oral corticosteroids (prednisone or budesonide), and/or antibiotics for their Crohn's disease [see Clinical Studies (14.4)].
The overall safety profile of ustekinumab was consistent with the safety profile seen in the adult psoriasis and psoriatic arthritis clinical trials. Common adverse reactions in trials CD-1 and CD-2 and in trial CD-3 are listed in Tables 6 and 7, respectively.
| Placebo N = 466 | Ustekinumab 6 mg/kg single intravenous induction dose N = 470 |
|
|---|---|---|
| Vomiting | 3% | 4% |
Other less common adverse reactions reported in subjects in trials CD-1 and CD-2 included asthenia (1% vs 0.4%), acne (1% vs 0.4%), and pruritus (2% vs 0.4%).
| Placebo | Ustekinumab 90 mg subcutaneous maintenance dose every 8 weeks |
|
|---|---|---|
| N = 133 | N = 131 | |
| Nasopharyngitis | 8% | 11% |
| Injection site erythema | 0 | 5% |
| Vulvovaginal candidiasis/mycotic infection | 1% | 5% |
| Bronchitis | 3% | 5% |
| Pruritus | 2% | 4% |
| Urinary tract infection | 2% | 4% |
| Sinusitis | 2% | 3% |
Infections
In subjects with Crohn's disease, serious or other clinically significant infections included anal abscess, gastroenteritis, and pneumonia. In addition, listeria meningitis and ophthalmic herpes zoster were reported in one patient each [see Warnings and Precautions (5.1)].
Malignancies
With up to one year of treatment in the Crohn's disease clinical trials, 0.2% of ustekinumab-treated subjects (0.36 events per hundred patient-years) and 0.2% of placebo-treated subjects (0.58 events per hundred patient-years) developed non-melanoma skin cancer. Malignancies other than non-melanoma skin cancers occurred in 0.2% of ustekinumab-treated subjects (0.27 events per hundred patient-years) and in none of the placebo-treated subjects.
Hypersensitivity Reactions Including Anaphylaxis
In CD trials, two subjects reported hypersensitivity reactions following ustekinumab administration. One patient experienced signs and symptoms consistent with anaphylaxis (tightness of the throat, shortness of breath, and flushing) after a single subcutaneous administration (0.1% of subjects receiving subcutaneous ustekinumab). In addition, one subject experienced signs and symptoms consistent with or related to a hypersensitivity reaction (chest discomfort, flushing, urticaria, and increased body temperature) after the initial intravenous ustekinumab dose (0.08% of subjects receiving intravenous ustekinumab). These subjects were treated with oral antihistamines or corticosteroids and in both cases, symptoms resolved within an hour.
Ulcerative Colitis
The safety of ustekinumab was evaluated in two randomized, double-blind, placebo-controlled clinical trials (UC-1 [IV induction] and UC-2 [SC maintenance]) in 960 adult subjects with moderately to severely active ulcerative colitis [see Clinical Studies (14.5)]. The overall safety profile of ustekinumab in subjects with ulcerative colitis was consistent with the safety profile seen across all approved indications. Adverse reactions reported in at least 3% of ustekinumab-treated subjects and at a higher rate than placebo were:
- Induction (UC-1): nasopharyngitis (7% vs 4%).
- Maintenance (UC-2): nasopharyngitis (24% vs 20%), headache (10% vs 4%), abdominal pain (7% vs 3%), influenza (6% vs 5%), fever (5% vs 4%), diarrhea (4% vs 1%), sinusitis (4% vs 1%), fatigue (4% vs 2%), and nausea (3% vs 2%).
Infections
In subjects with ulcerative colitis, serious or other clinically significant infections included gastroenteritis and pneumonia. In addition, listeriosis and ophthalmic herpes zoster were reported in one subject each [see Warnings and Precautions (5.1)].
Malignancies
With up to one year of treatment in the ulcerative colitis clinical trials, 0.4% of ustekinumab-treated subjects (0.48 events per hundred patient-years) and 0.0% of placebo-treated subjects (0.00 events per hundred patient-years) developed non-melanoma skin cancer. Malignancies other than non-melanoma skin cancers occurred in 0.5% of ustekinumab-treated subjects (0.64 events per hundred patient-years) and 0.2% of placebo-treated subjects (0.40 events per hundred patient-years).
6.2 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of ustekinumab or of other ustekinumab products.
Approximately 6 to 12.4% of subjects treated with ustekinumab in plaque psoriasis and psoriatic arthritis clinical trials developed antibodies to ustekinumab, which were generally low-titer. In plaque psoriasis clinical trials, antibodies to ustekinumab were associated with reduced or undetectable serum ustekinumab concentrations and reduced efficacy. In plaque psoriasis trials, the majority of subjects who were positive for antibodies to ustekinumab had neutralizing antibodies.
In Crohn's disease and ulcerative colitis clinical trials, 2.9% and 4.6% of subjects, respectively, developed antibodies to ustekinumab when treated with ustekinumab for approximately one year. No apparent association between the development of antibodies to ustekinumab and the development of injection site reactions was seen.
6.3 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of ustekinumab products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to ustekinumab product exposure.
Immune system disorders: Serious hypersensitivity reactions (including anaphylaxis and angioedema), other hypersensitivity reactions (including rash and urticaria).
Infections and infestations: Lower respiratory tract infection (including opportunistic fungal infections and tuberculosis).
Neurological disorders: Posterior Reversible Encephalopathy Syndrome (PRES).
Respiratory, thoracic and mediastinal disorders: Interstitial pneumonia, eosinophilic pneumonia and cryptogenic organizing pneumonia.
Skin reactions: Pustular psoriasis, erythrodermic psoriasis, hypersensitivity vasculitis.
7 DRUG INTERACTIONS
7.1 Concomitant Therapies
In plaque psoriasis trials the safety of ustekinumab products in combination with immunosuppressive agents or phototherapy has not been evaluated. In psoriatic arthritis trials, concomitant MTX use did not appear to influence the safety or efficacy of ustekinumab. In Crohn's disease and ulcerative colitis induction trials, immunomodulators (6-MP, AZA, MTX) were used concomitantly in approximately 30% of subjects and corticosteroids were used concomitantly in approximately 40% and 50% of Crohn's disease and ulcerative colitis subjects, respectively. Use of these concomitant therapies did not appear to influence the overall safety or efficacy of ustekinumab.
7.2 CYP450 Substrates
The formation of CYP450 enzymes can be suppressed by increased levels of certain cytokines (e.g., IL-1, IL-6, TNFα, IFN) during chronic inflammation. Thus, use of ustekinumab products, antagonists of IL-12 and IL-23, could normalize the formation of CYP450 enzymes. Upon initiation or discontinuation of WEZLANA in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index, consider monitoring for therapeutic effect or drug concentration and adjust the individual dosage of the CYP substrate as needed. See the prescribing information of specific CYP substrates.
A CYP-mediated drug interaction effect was not observed in subjects with Crohn's disease [see Clinical Pharmacology (12.3)].
7.3 Allergen Immunotherapy
Ustekinumab products have not been evaluated in patients who have undergone allergy immunotherapy. Ustekinumab products may decrease the protective effect of allergen immunotherapy (decrease tolerance) which may increase the risk of an allergic reaction to a dose of allergen immunotherapy. Therefore, caution should be exercised in patients receiving or who have received allergen immunotherapy, particularly for anaphylaxis.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data from observational studies, published case reports, and postmarketing surveillance on the use of ustekinumab products during pregnancy are insufficient to inform a drug associated risk of major birth defects, miscarriage, and other adverse maternal or fetal outcomes. Transport of human IgG antibody across the placenta increases as pregnancy progresses and peaks during the third trimester; therefore, ustekinumab products may be transferred to the developing fetus [see Clinical Considerations]. In animal reproductive and developmental toxicity studies, no adverse developmental effects were observed in offspring after administration of ustekinumab to pregnant monkeys at exposures greater than 100 times the maximum recommended human dose (MRHD).
The background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage of clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Because ustekinumab products may theoretically interfere with immune response to infections, consider risks and benefits prior to administering live vaccines to infants exposed to WEZLANA in utero. There are insufficient data regarding exposed infant serum levels of ustekinumab products at birth and the duration of persistence of ustekinumab products in infant serum after birth. Although a specific timeframe to delay administration of live attenuated vaccines in infants exposed in utero is unknown, consider the risks and benefits of delaying a minimum of 6 months after birth because of the clearance of the product.
Data
Animal Data
Ustekinumab was tested in two embryo-fetal development toxicity studies in cynomolgus monkeys. No teratogenic or other adverse developmental effects were observed in fetuses from pregnant monkeys that were administered ustekinumab subcutaneously twice weekly or intravenously weekly during the period of organogenesis. Serum concentrations of ustekinumab in pregnant monkeys were greater than 100 times the serum concentration in patients treated subcutaneously with 90 mg of ustekinumab weekly for 4 weeks.
In a combined embryo-fetal development and pre- and post-natal development toxicity study, pregnant cynomolgus monkeys were administered subcutaneous doses of ustekinumab twice weekly at exposures greater than 100 times the MRHD from the beginning of organogenesis to Day 33 after delivery. Neonatal deaths occurred in the offspring of one monkey administered ustekinumab at 22.5 mg/kg and one monkey dosed at 45 mg/kg. No ustekinumab-related effects on functional, morphological, or immunological development were observed in the neonates from birth through six months of age.
8.2 Lactation
Risk Summary
Limited data from published literature suggests that ustekinumab is present in human breast milk. There are no available data on the effects of ustekinumab products on milk production. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to ustekinumab products are unknown. No adverse effects on the breastfed infant causally related to ustekinumab products have been identified in the published literature or postmarketing experience.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for WEZLANA and any potential adverse effects on the breastfed child from WEZLANA or from the underlying maternal condition.
8.4 Pediatric Use
Plaque Psoriasis
The safety and effectiveness of WEZLANA have been established for the treatment of moderate to severe plaque psoriasis in pediatric patients 6 to 17 years of age who are candidates for phototherapy or systemic therapy.
Use of WEZLANA in pediatric patients 12 to less than 17 years of age is supported by evidence from a multicenter, randomized, 60-week trial (Ps STUDY 3) of ustekinumab that included a 12-week, double-blind, placebo-controlled, parallel-group portion, in 110 pediatric subjects 12 years of age and older [see Adverse Reactions (6.1), Clinical Studies (14.2)].
Use of WEZLANA in pediatric patients 6 to 11 years of age is supported by evidence from an open-label, single-arm, efficacy, safety, and pharmacokinetics trial (Ps STUDY 4) of ustekinumab in 44 subjects [see Adverse Reactions (6.1), Pharmacokinetics (12.3)].
The safety and effectiveness of WEZLANA have not been established in pediatric patients less than 6 years of age with plaque psoriasis.
Psoriatic Arthritis
The safety and effectiveness of WEZLANA have been established for treatment of psoriatic arthritis in pediatric patients 6 to 17 years old.
Use of WEZLANA in these age groups is supported by evidence from adequate and well controlled trials of ustekinumab in adults with psoriasis and PsA, pharmacokinetic data from adult subjects with psoriasis, adult subjects with PsA and pediatric subjects with psoriasis, and safety data of ustekinumab from two clinical trials in 44 pediatric subjects 6 to 11 years old with psoriasis and 110 pediatric subjects 12 to 17 years old with psoriasis. The observed pre-dose (trough) concentrations are generally comparable between adult subjects with psoriasis, adult subjects with PsA and pediatric subjects with psoriasis, and the PK exposure is expected to be comparable between adult and pediatric subjects with PsA [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1, 14.2, 14.3)].
The safety and effectiveness of WEZLANA have not been established in pediatric patients less than 6 years old with psoriatic arthritis.
8.5 Geriatric Use
Of the 6709 subjects exposed to ustekinumab, a total of 340 were 65 years of age or older (183 subjects with plaque psoriasis, 65 subjects with psoriatic arthritis, 58 subjects with Crohn's disease and 34 subjects with ulcerative colitis), and 40 subjects were 75 years of age or older. Clinical trials of ustekinumab did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
10 OVERDOSAGE
Single doses up to 6 mg/kg intravenously have been administered in clinical trials without dose-limiting toxicity. In case of overdosage, monitor the patient for any signs or symptoms of adverse reactions or effects and institute appropriate symptomatic treatment immediately. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
11 DESCRIPTION
Ustekinumab-auub, a human IgG1κ monoclonal antibody, is a human interleukin -12 and -23 antagonist. Using DNA recombinant technology, ustekinumab-auub is produced in a mammalian cell line (Chinese Hamster Ovary). The manufacturing process contains steps for the clearance of viruses. Ustekinumab-auub is comprised of 1326 amino acids and has an estimated molecular mass that ranges from 148 to 150 kDa.
WEZLANA (ustekinumab-auub) injection is a sterile, preservative-free, clear to opalescent and colorless to light yellow solution with a pH of 6.0.
WEZLANA for Subcutaneous Use
Available as 45 mg of ustekinumab-auub in 0.5 mL and 90 mg of ustekinumab-auub in 1 mL, supplied as a sterile solution in a single-dose prefilled syringe with a 27 gauge fixed ½ inch needle and a single-dose prefilled ConfiPen autoinjector, and as 45 mg of ustekinumab-auub in 0.5 mL in a single-dose Type I glass vial with a coated stopper. The syringe is fitted with a passive needle guard and a needle cap that does not contain dry natural rubber (a derivative of latex). The prefilled ConfiPen autoinjector is not made with natural rubber latex.
Each 0.5 mL prefilled syringe, prefilled ConfiPen autoinjector or vial delivers 45 mg ustekinumab-auub, histidine (0.23 mg) and histidine hydrochloride monohydrate (0.36 mg), Polysorbate 80 (0.02 mg), and sucrose (38 mg).
Each 1 mL prefilled syringe, or prefilled ConfiPen autoinjector delivers 90 mg ustekinumab-auub, histidine (0.46 mg) and histidine hydrochloride monohydrate (0.72 mg), Polysorbate 80 (0.04 mg), and sucrose (76 mg).
WEZLANA for Intravenous Infusion
Available as 130 mg of ustekinumab-auub in 26 mL, supplied as a single-dose Type I glass vial with a coated stopper.
Each 26 mL vial delivers 130 mg ustekinumab-auub, edetate disodium (0.47 mg), histidine (20 mg), histidine hydrochloride monohydrate (27 mg), methionine (10.4 mg), Polysorbate 80 (10.4 mg) and sucrose (2210 mg).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ustekinumab products are human IgG1κ monoclonal antibodies that bind with specificity to the p40 protein subunit used by both the IL-12 and IL-23 cytokines. IL-12 and IL-23 are naturally occurring cytokines that are involved in inflammatory and immune responses, such as natural killer cell activation and CD4+ T -cell differentiation and activation. In in vitro models, ustekinumab products were shown to disrupt IL-12 and IL-23 mediated signaling and cytokine cascades by disrupting the interaction of these cytokines with a shared cell-surface receptor chain, IL-12Rβ1. The cytokines IL-12 and IL-23 have been implicated as important contributors to the chronic inflammation that is a hallmark of Crohn's disease and ulcerative colitis. In animal models of colitis, genetic absence or antibody blockade of the p40 subunit of IL-12 and IL-23, the target of ustekinumab products, was shown to be protective.
12.2 Pharmacodynamics
Plaque Psoriasis
In a small exploratory trial, a decrease was observed in the expression of mRNA of its molecular targets IL-12 and IL-23 in lesional skin biopsies measured at baseline and up to two weeks post-treatment in subjects with plaque psoriasis.
Ulcerative Colitis
In both trial UC-1 (induction) and trial UC-2 (maintenance), a positive relationship was observed between exposure and rates of clinical remission, clinical response, and endoscopic improvement. The response rate approached a plateau at the ustekinumab exposures associated with the recommended dosing regimen for maintenance treatment [see Clinical Studies (14.5)].
12.3 Pharmacokinetics
Absorption
In adult subjects with plaque psoriasis, the median time to reach the maximum serum concentration (Tmax) was 13.5 days and 7 days, respectively, after a single subcutaneous administration of 45 mg (N = 22) and 90 mg (N = 24) of ustekinumab. In healthy subjects (N = 30), the median Tmax value (8.5 days) following a single subcutaneous administration of 90 mg of ustekinumab was comparable to that observed in subjects with plaque psoriasis.
Following multiple subcutaneous doses of ustekinumab in adult subjects with plaque psoriasis, steady-state serum concentrations of ustekinumab were achieved by Week 28. The mean (±SD) steady-state trough serum ustekinumab concentrations were 0.69 ± 0.69 mcg/mL for subjects less than or equal to 100 kg receiving a 45 mg dose and 0.74 ± 0.78 mcg/mL for subjects greater than 100 kg receiving a 90 mg dose. There was no apparent accumulation in serum ustekinumab concentration over time when given subcutaneously every 12 weeks.
Following the recommended intravenous induction dose, mean ±SD peak serum ustekinumab concentration was 125.2 ± 33.6 mcg/mL in subjects with Crohn's disease, and 129.1 ± 27.6 mcg/mL in subjects with ulcerative colitis. Starting at Week 8, the recommended subcutaneous maintenance dosing of 90 mg ustekinumab was administered every 8 weeks. Steady-state ustekinumab concentration was achieved by the start of the second maintenance dose. There was no apparent accumulation in ustekinumab concentration over time when given subcutaneously every 8 weeks. Mean ±SD steady-state trough concentration was 2.5 ± 2.1 mcg/mL in subjects with Crohn's disease, and 3.3 ± 2.3 mcg/mL in subjects with ulcerative colitis for 90 mg ustekinumab administered every 8 weeks.
Distribution
Population pharmacokinetic analyses showed that the volume of distribution of ustekinumab in the central compartment was 2.7 L (95% CI: 2.69, 2.78) in subjects with Crohn's disease and 3.0 L (95% CI: 2.96, 3.07) in subjects with ulcerative colitis. The total volume of distribution at steady-state was 4.6 L in subjects with Crohn's disease and 4.4 L in subjects with ulcerative colitis.
Elimination
The mean (±SD) half-life ranged from 14.9 ± 4.6 to 45.6 ± 80.2 days across all plaque psoriasis trials following subcutaneous administration. Population pharmacokinetic analyses showed that the clearance of ustekinumab was 0.19 L/day (95% CI: 0.185, 0.197) in subjects with Crohn's disease and 0.19 L/day (95% CI: 0.179, 0.192) in subjects with ulcerative colitis with an estimated median terminal half-life of approximately 19 days for both IBD (Crohn's disease and ulcerative colitis) populations.
These results indicate the pharmacokinetics of ustekinumab were similar between subjects with Crohn's disease and ulcerative colitis.
Metabolism
The metabolic pathway of ustekinumab products has not been characterized. As a human IgG1κ monoclonal antibody, ustekinumab products are expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Specific Populations
Weight
When given the same dose, subjects with plaque psoriasis or psoriatic arthritis weighing more than 100 kg had lower median serum ustekinumab concentrations compared with those subjects weighing 100 kg or less. The median trough serum concentrations of ustekinumab in subjects of higher weight (greater than 100 kg) in the 90 mg group were comparable to those in subjects of lower weight (100 kg or less) in the 45 mg group.
Age: Geriatric Population
A population pharmacokinetic analysis (N = 106/1937 subjects with plaque psoriasis greater than or equal to 65 years old) was performed to evaluate the effect of age on the pharmacokinetics of ustekinumab. There were no apparent changes in pharmacokinetic parameters (clearance and volume of distribution) in subjects older than 65 years old.
Age: Pediatric Population
Following multiple recommended doses of ustekinumab in pediatric subjects 6 to 17 years of age with plaque psoriasis, steady-state serum concentrations of ustekinumab were achieved by Week 28. At Week 28, the mean ±SD steady-state trough serum ustekinumab concentrations were 0.36 ± 0.26 mcg/mL and 0.54 ± 0.43 mcg/mL, respectively, in pediatric subjects 6 to 11 years of age and pediatric subjects 12 to 17 years of age.
Overall, the observed steady-state ustekinumab trough concentrations in pediatric subjects with plaque psoriasis were within the range of those observed for adult subjects with plaque psoriasis and adult subjects with PsA after administration of ustekinumab.
Drug Interaction Studies
The effects of IL-12 or IL-23 on the regulation of CYP450 enzymes were evaluated in an in vitro study using human hepatocytes, which showed that IL-12 and/or IL-23 at levels of 10 ng/mL did not alter human CYP450 enzyme activities (CYP1A2, 2B6, 2C9, 2C19, 2D6, or 3A4).
No clinically significant changes in exposure of caffeine (CYP1A2 substrate), warfarin (CYP2C9 substrate), omeprazole (CYP2C19 substrate), dextromethorphan (CYP2D6 substrate), or midazolam (CYP3A substrate) were observed when used concomitantly with ustekinumab at the approved recommended dosage in subjects with Crohn's disease [see Drug Interactions (7.2)].
Population pharmacokinetic analyses indicated that the clearance of ustekinumab was not impacted by concomitant MTX, NSAIDs, and oral corticosteroids, or prior exposure to a TNF blocker in subjects with psoriatic arthritis.
In subjects with Crohn's disease and ulcerative colitis, population pharmacokinetic analyses did not indicate changes in ustekinumab clearance with concomitant use of corticosteroids or immunomodulators (AZA, 6-MP, or MTX); and serum ustekinumab concentrations were not impacted by concomitant use of these medications.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of ustekinumab products. Published literature showed that administration of murine IL-12 caused an anti-tumor effect in mice that contained transplanted tumors and IL-12/IL-23p40 knockout mice or mice treated with anti-IL-12/IL-23p40 antibody had decreased host defense to tumors. Mice genetically manipulated to be deficient in both IL-12 and IL-23 or IL-12 alone developed UV-induced skin cancers earlier and more frequently compared to wild-type mice. The relevance of these experimental findings in mouse models for malignancy risk in humans is unknown.
No effects on fertility were observed in male cynomolgus monkeys that were administered ustekinumab at subcutaneous doses up to 45 mg/kg twice weekly (45 times the MRHD on a mg/kg basis) prior to and during the mating period. However, fertility and pregnancy outcomes were not evaluated in mated females.
No effects on fertility were observed in female mice that were administered an analogous IL-12/IL- 23p40 antibody by subcutaneous administration at doses up to 50 mg/kg, twice weekly, prior to and during early pregnancy.
14 CLINICAL STUDIES
14.1 Adult Plaque Psoriasis
Two multicenter, randomized, double-blind, placebo-controlled trials (Ps STUDY 1 and Ps STUDY 2) enrolled a total of 1996 subjects 18 years of age and older with plaque psoriasis who had a minimum body surface area involvement of 10%, and Psoriasis Area and Severity Index (PASI) score ≥ 12, and who were candidates for phototherapy or systemic therapy. Subjects with guttate, erythrodermic, or pustular psoriasis were excluded from the trials.
Ps STUDY 1 enrolled 766 subjects and Ps STUDY 2 enrolled 1230 subjects. The trials had the same design through Week 28. In both trials, subjects were randomized in equal proportion to placebo, 45 mg or 90 mg of ustekinumab. Subjects randomized to ustekinumab received 45 mg or 90 mg doses, regardless of weight, at Weeks 0, 4, and 16. Subjects randomized to receive placebo at Weeks 0 and 4 crossed over to receive ustekinumab (either 45 mg or 90 mg) at Weeks 12 and 16.
In both trials, subjects in all treatment groups had a median baseline PASI score ranging from approximately 17 to 18. Baseline PGA score was marked or severe in 44% of subjects in Ps STUDY 1 and 40% of subjects in Ps STUDY 2. Approximately two-thirds of all subjects had received prior phototherapy, 69% had received either prior conventional systemic or biologic therapy for the treatment of psoriasis, with 56% receiving prior conventional systemic therapy and 43% receiving prior biologic therapy. A total of 28% of subjects had a history of psoriatic arthritis.
In both trials, the endpoints were the proportion of subjects who achieved at least a 75% reduction in PASI score (PASI 75) from baseline to Week 12 and treatment success (cleared or minimal) on the Physician's Global Assessment (PGA). The PGA is a 6-category scale ranging from 0 (cleared) to 5 (severe) that indicates the physician's overall assessment of psoriasis focusing on plaque thickness/induration, erythema, and scaling.
Clinical Response
The results of Ps STUDY 1 and Ps STUDY 2 are presented in Table 8 below.
| Ps STUDY 1 | Ps STUDY 2 | |||||
|---|---|---|---|---|---|---|
| Placebo | ustekinumab | Placebo | ustekinumab | |||
| 45 mg | 90 mg | 45 mg | 90 mg | |||
| Subjects randomized | 255 | 255 | 256 | 410 | 409 | 411 |
| PASI 75 response | 8 (3%) | 171 (67%) | 170 (66%) | 15 (4%) | 273 (67 %) | 311 (76%) |
| PGA of Cleared or Minimal | 10 (4%) | 151 (59%) | 156 (61%) | 18 (4%) | 277 (68%) | 300 (73%) |
Examination of age, gender, and race subgroups did not identify differences in response to ustekinumab among these subgroups.
In subjects who weighed 100 kg or less, response rates were comparable with both the 45 mg and 90 mg doses; however, in subjects who weighed greater than 100 kg, higher response rates were seen with 90 mg dosing compared with 45 mg dosing (Table 9 below).
| Ps STUDY 1 | Ps STUDY 2 | |||||
|---|---|---|---|---|---|---|
| ustekinumab | ustekinumab | |||||
| Placebo | 45 mg | 90 mg | Placebo | 45 mg | 90 mg | |
|
|
||||||
| Subjects randomized | 255 | 255 | 256 | 410 | 409 | 411 |
| PASI 75 response* | ||||||
| ≤ 100 kg | 4% | 74% | 65% | 4% | 73% | 78% |
| 6/166 | 124/168 | 107/164 | 12/290 | 218/297 | 225/289 | |
| > 100 kg | 2% | 54% | 68% | 3% | 49% | 71% |
| 2/89 | 47/87 | 63/92 | 3/120 | 55/112 | 86/121 | |
| PGA of Cleared or Minimal | ||||||
| ≤ 100 kg | 4% | 64% | 63% | 5% | 74% | 75% |
| 7/166 | 108/168 | 103/164 | 14/290 | 220/297 | 216/289 | |
| > 100 kg | 3% | 49% | 58% | 3% | 51% | 69% |
| 3/89 | 43/87 | 53/92 | 4/120 | 57/112 | 84/121 | |
Subjects in Ps STUDY 1 who were PASI 75 responders at both Weeks 28 and 40 were re-randomized at Week 40 to either continued dosing of ustekinumab (ustekinumab at Week 40) or to withdrawal of therapy (placebo at Week 40). At Week 52, 89% (144/162) of subjects re-randomized to ustekinumab treatment were PASI 75 responders compared with 63% (100/159) of subjects re-randomized to placebo (treatment withdrawal after Week 28 dose). The median time to loss of PASI 75 response among the subjects randomized to treatment withdrawal was 16 weeks.
14.2 Pediatric Plaque Psoriasis
A multicenter, randomized, double blind, placebo-controlled trial (Ps STUDY 3) enrolled 110 pediatric subjects 12 to 17 years of age with a minimum BSA involvement of 10%, a PASI score greater than or equal to 12, and a PGA score greater than or equal to 3, who were candidates for phototherapy or systemic therapy and whose disease was inadequately controlled by topical therapy.
Subjects were randomized to receive placebo (n = 37), the recommended dose of ustekinumab (n = 36), or one -half the recommended dose of ustekinumab (n = 37) by subcutaneous injection at Weeks 0 and 4 followed by dosing every 12 weeks (q12w). The recommended dose of ustekinumab was 0.75 mg/kg for subjects weighing less than 60 kg, 45 mg for subjects weighing 60 kg to 100 kg, and 90 mg for subjects weighing greater than 100 kg. At Week 12, subjects who received placebo were crossed over to receive ustekinumab at the recommended dose or one -half the recommended dose.
Of the pediatric subjects, approximately 63% had prior exposure to phototherapy or conventional systemic therapy and approximately 11% had prior exposure to biologics.
The endpoints were the proportion of subjects who achieved a PGA score of cleared (0) or minimal (1), PASI 75, and PASI 90 at Week 12. Subjects were followed for up to 60 weeks following first administration of trial agent.
Clinical Response
The efficacy results at Week 12 for Ps STUDY 3 are presented in Table 10.
| Ps STUDY 3 | ||
|---|---|---|
| Placebo n (%) | ustekinumab*
n (%) |
|
|
|
||
| N | 37 | 36 |
| PGA | ||
| PGA of cleared (0) or minimal (1) | 2 (5.4%) | 25 (69.4%) |
| PASI | ||
| PASI 75 responders | 4 (10.8%) | 29 (80.6%) |
| PASI 90 responders | 2 (5.4%) | 22 (61.1%) |
14.3 Psoriatic Arthritis
The safety and efficacy of ustekinumab was assessed in 927 subjects (PsA STUDY 1, n = 615; PsA STUDY 2, n = 312), in two randomized, double-blind, placebo-controlled trials in adult subjects 18 years of age and older with active PsA (≥ 5 swollen joints and ≥ 5 tender joints) despite non-steroidal anti-inflammatory (NSAID) or disease modifying antirheumatic (DMARD) therapy. Subjects in these trials had a diagnosis of PsA for at least 6 months. Subjects with each subtype of PsA were enrolled, including polyarticular arthritis with the absence of rheumatoid nodules (39%), spondylitis with peripheral arthritis (28%), asymmetric peripheral arthritis (21%), distal interphalangeal involvement (12%) and arthritis mutilans (0.5%). Over 70% and 40% of the subjects, respectively, had enthesitis and dactylitis at baseline.
Subjects were randomized to receive treatment with ustekinumab 45 mg, 90 mg, or placebo subcutaneously at Weeks 0 and 4 followed by every 12 weeks (q12w) dosing. Approximately 50% of subjects continued on stable doses of MTX (≤ 25 mg/week). The primary endpoint was the percentage of subjects achieving ACR 20 response at Week 24. In PsA STUDY 1 and PsA STUDY 2, 80% and 86% of the subjects, respectively, had been previously treated with DMARDs. In PsA STUDY 1, previous treatment with anti-tumor necrosis factor (TNF)-α agent was not allowed. In PsA STUDY 2, 58% (n = 180) of the subjects had been previously treated with TNF blocker, of whom over 70% had discontinued their TNF blocker treatment for lack of efficacy or intolerance at any time.
Clinical Response
In both trials, a greater proportion of subjects achieved ACR 20, ACR 50 and PASI 75 response in the ustekinumab 45 mg and 90 mg groups compared to placebo at Week 24 (see Table 11). ACR 70 responses were also higher in the ustekinumab 45 mg and 90 mg groups, although the difference was only numerical (p = NS) in STUDY 2. Responses were consistent in subjects treated with ustekinumab alone or in combination with methotrexate. Responses were similar in subjects regardless of prior TNFα exposure.
| PsA STUDY 1 ustekinumab | PsA STUDY 2 ustekinumab |
|||||
|---|---|---|---|---|---|---|
| Placebo | 45 mg | 90 mg | Placebo | 45 mg | 90 mg | |
|
|
||||||
| Number of subjects randomized | 206 | 205 | 204 | 104 | 103 | 105 |
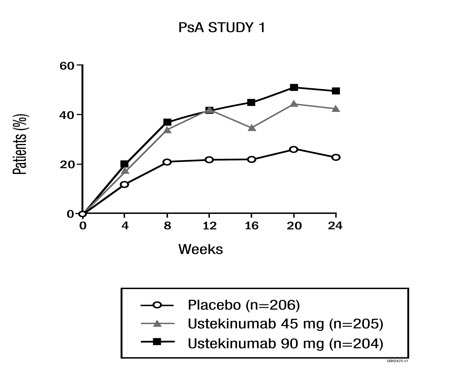
| ACR 20 response, N (%) | 47 (23%) | 87 (42%) | 101 (50%) | 21 (20%) | 45 (44%) | 46 (44%) |
| ACR 50 response, N (%) | 18 (9%) | 51 (25%) | 57 (28%) | 7 (7%) | 18 (17%) | 24 (23%) |
| ACR 70 response, N (%) | 5 (2%) | 25 (12%) | 29 (14%) | 3 (3%) | 7 (7%) | 9 (9%) |
| Number of subjects with ≥ 3% BSA* | 146 | 145 | 149 | 80 | 80 | 81 |
| PASI 75 response, N (%) | 16 (11%) | 83 (57%) | 93 (62%) | 4 (5%) | 41 (51%) | 45 (56%) |
The percent of subjects achieving ACR 20 responses by visit is shown in Figure 1.
| Figure 1. Percent of subjects achieving ACR 20 response through Week 24 |
|---|
 |
The results of the components of the ACR response criteria are shown in Table 12.
| PsA STUDY 1 | |||
|---|---|---|---|
| Placebo | ustekinumab | ||
| (N = 206) | 45 mg (N = 205) | 90 mg (N = 204) |
|
|
|
|||
| Number of swollen joints* | |||
| Baseline | 15 | 12 | 13 |
| Mean Change at Week 24 | -3 | -5 | -6 |
| Number of tender joints† | |||
| Baseline | 25 | 22 | 23 |
| Mean Change at Week 24 | -4 | -8 | -9 |
| Subjects' assessment of pain‡ | |||
| Baseline | 6.1 | 6.2 | 6.6 |
| Mean Change at Week 24 | -0.5 | -2.0 | -2.6 |
| Subject global assessment‡ | |||
| Baseline | 6.1 | 6.3 | 6.4 |
| Mean Change at Week 24 | -0.5 | -2.0 | -2.5 |
| Physician global assessment‡ | |||
| Baseline | 5.8 | 5.7 | 6.1 |
| Mean Change at Week 24 | -1.4 | -2.6 | -3.1 |
| Disability index (HAQ)§ | |||
| Baseline | 1.2 | 1.2 | 1.2 |
| Mean Change at Week 24 | -0.1 | -0.3 | -0.4 |
| CRP (mg/dL)¶ | |||
| Baseline | 1.6 | 1.7 | 1.8 |
| Mean Change at Week 24 | 0.01 | -0.5 | -0.8 |
An improvement in enthesitis and dactylitis scores was observed in each ustekinumab group compared with placebo at Week 24.
Physical Function
Ustekinumab-treated subjects showed improvement in physical function compared to subjects treated with placebo as assessed by HAQ-DI at Week 24. In both trials, the proportion of HAQ-DI responders (≥ 0.3 improvement in HAQ-DI score) was greater in the ustekinumab 45 mg and 90 mg groups compared to placebo at Week 24.
14.4 Crohn's Disease
Ustekinumab was evaluated in three randomized, double-blind, placebo-controlled clinical trials in adult subjects with moderately to severely active Crohn's disease (Crohn's Disease Activity Index [CDAI] score of 220 to 450). There were two 8-week intravenous induction trials (CD-1 and CD-2) followed by a 44-week subcutaneous randomized withdrawal maintenance trial (CD-3) representing 52 weeks of therapy. Subjects in CD-1 had failed or were intolerant to treatment with one or more TNF blockers, while subjects in CD-2 had failed or were intolerant to treatment with immunomodulators or corticosteroids, but never failed treatment with a TNF blocker.
Trials CD-1 and CD-2
In trials CD-1 and CD-2, 1409 subjects were randomized, of whom 1368 (CD-1, n = 741; CD-2, n = 627) were included in the final efficacy analysis. Induction of clinical response (defined as a reduction in CDAI score of greater than or equal to 100 points or CDAI score of less than 150) at Week 6 and clinical remission (defined as a CDAI score of less than 150) at Week 8 were evaluated. In both trials, subjects were randomized to receive a single intravenous administration of ustekinumab at either approximately 6 mg/kg, placebo (see Table 4), or 130 mg (a lower dose than recommended).
In trial CD-1, subjects had failed or were intolerant to prior treatment with a TNF blocker: 29% subjects had an inadequate initial response (primary non-responders), 69% responded but subsequently lost response (secondary non-responders) and 36% were intolerant to a TNF blocker. Of these subjects, 48% failed or were intolerant to one TNF blocker and 52% had failed 2 or 3 prior TNF blockers. At baseline and throughout the trial, approximately 46% of the subjects were receiving corticosteroids and 31% of the subjects were receiving immunomodulators (AZA, 6-MP, MTX). The median baseline CDAI score was 319 in the ustekinumab approximately 6 mg/kg group and 313 in the placebo group.
In trial CD-2, subjects had failed or were intolerant to prior treatment with corticosteroids (81% of subjects), at least one immunomodulator (6-MP, AZA, MTX; 68% of subjects), or both (49% of subjects). Additionally, 69% never received a TNF blocker and 31% previously received but had not failed a TNF blocker. At baseline, and throughout the trial, approximately 39% of the subjects were receiving corticosteroids and 35% of the subjects were receiving immunomodulators (AZA, 6-MP, MTX). The median baseline CDAI score was 286 in the ustekinumab and 290 in the placebo group.
In these induction trials, a greater proportion of subjects treated with ustekinumab (at the recommended dose of approximately 6 mg/kg dose) achieved clinical response at Week 6 and clinical remission at Week 8 compared to placebo (see Table 13 for clinical response and remission rates).
Clinical response and remission were significant as early as Week 3 in ustekinumab-treated subjects and continued to improve through Week 8.
| CD-1 n = 741 | CD-2 n = 627 | |||||
|---|---|---|---|---|---|---|
| Placebo N = 247 | Ustekinumab‡
N = 249 | Treatment difference and 95% CI | Placebo N = 209 | Ustekinumab‡
N = 209 | Treatment difference and 95% CI | |
| Clinical remission is defined as CDAI score < 150; Clinical response is defined as reduction in CDAI score by at least 100 points or being in clinical remission: 70 point response is defined as reduction in CDAI score by at least 70 points | ||||||
|
|
||||||
| Clinical Response (100 point), Week 6 | 53 (21%) | 84 (34%)§ | 12% (4%, 20%) | 60 (29%) | 116 (56%)¶ | 27% (18%, 36%) |
| Clinical Remission, Week 8 | 18 (7%) | 52 (21%)¶ | 14% (8%, 20%) | 41 (20%) | 84 (40%)¶ | 21% (12%, 29%) |
| Clinical Response (100 point), Week 8 | 50 (20%) | 94 (38%)¶ | 18% (10%, 25%) | 67 (32%) | 121 (58%)¶ | 26% (17%, 35%) |
| 70 Point Response, Week 6 | 75 (30%) | 109 (44%)§ | 13% (5%, 22%) | 81 (39%) | 135 (65%)¶ | 26% (17%, 35%) |
| 70 Point Response, Week 3 | 67 (27%) | 101 (41%)§ | 13% (5%, 22%) | 66 (32%) | 106 (51%)¶ | 19% (10%, 28%) |
Trial CD-3
The maintenance trial (CD-3) evaluated 388 subjects who achieved clinical response (≥ 100 point reduction in CDAI score) at Week 8 with either induction dose of ustekinumab in trials CD-1 or CD-2. Subjects were randomized to receive a subcutaneous maintenance regimen of either 90 mg ustekinumab every 8 weeks or placebo for 44 weeks (see Table 14).
| Placebo*
N = 131† | 90 mg ustekinumab every 8 weeks N = 128† | Treatment difference and 95% CI | |
|---|---|---|---|
| Clinical remission is defined as CDAI score < 150; Clinical response is defined as reduction in CDAI of at least 100 points or being in clinical remission | |||
|
|
|||
| Clinical Remission | 47 (36%) | 68 (53%)‡ | 17% (5%,29%) |
| Clinical Response | 58 (44%) | 76 (59%)§ | 15% (3%,27%) |
| Clinical Remission in subjects in remission at the start of maintenance therapy¶ | 36/79 (46%) | 52/78 (67%)‡ | 21% (6%, 36%) |
At Week 44, 47% of subjects who received ustekinumab were corticosteroid-free and in clinical remission, compared to 30% of subjects in the placebo group.
At Week 0 of trial CD-3, 34/56 (61%) ustekinumab-treated subjects who previously failed or were intolerant to TNF blocker therapies were in clinical remission and 23/56 (41%) of these subjects were in clinical remission at Week 44. In the placebo arm, 27/61 (44%) subjects were in clinical remission at Week 0 while 16/61 (26%) of these subjects were in remission at Week 44.
At Week 0 of trial CD-3, 46/72 (64%) ustekinumab-treated subjects who had previously failed immunomodulator therapy or corticosteroids (but not TNF blockers) were in clinical remission and 45/72 (63%) of these subjects were in clinical remission at Week 44. In the placebo arm, 50/70 (71%) of these subjects were in clinical remission at Week 0 while 31/70 (44%) were in remission at Week 44. In the subset of these subjects who were also naïve to TNF blockers, 34/52 (65%) of ustekinumab-treated subjects were in clinical remission at Week 44 as compared to 25/51 (49%) in the placebo arm.
Subjects who were not in clinical response 8 weeks after ustekinumab induction were not included in the primary efficacy analyses for trial CD-3; however, these subjects were eligible to receive a 90 mg subcutaneous injection of ustekinumab upon entry into trial CD-3. Of these subjects, 102/219 (47%) achieved clinical response eight weeks later and were followed for the duration of the trial.
14.5 Ulcerative Colitis
Ustekinumab was evaluated in two randomized, double-blind, placebo-controlled clinical trials [UC-1 and UC-2 (NCT02407236)] in adult subjects with moderately to severely active ulcerative colitis who had an inadequate response to or failed to tolerate a biologic (i.e., TNF blocker and/or vedolizumab), corticosteroids, and/or 6-MP or AZA therapy. The 8-week intravenous induction trial (UC-1) was followed by the 44-week subcutaneous randomized withdrawal maintenance trial (UC-2) for a total of 52 weeks of therapy.
Disease assessment was based on the Mayo score, which ranged from 0 to 12 and has four subscores that were each scored from 0 (normal) to 3 (most severe): stool frequency, rectal bleeding, findings on centrally-reviewed endoscopy, and physician global assessment. Moderately to severely active ulcerative colitis was defined at baseline (Week 0) as Mayo score of 6 to 12, including a Mayo endoscopy subscore ≥ 2. An endoscopy score of 2 was defined by marked erythema, absent vascular pattern, friability, erosions; and a score of 3 was defined by spontaneous bleeding, ulceration. At baseline, subjects had a median Mayo score of 9, with 84% of subjects having moderate disease (Mayo score 6–10) and 15% having severe disease (Mayo score 11–12).
Subjects in these trials may have received other concomitant therapies including aminosalicylates, immunomodulatory agents (AZA, 6-MP, or MTX), and oral corticosteroids (prednisone).
Trial UC-1
In UC-1, 961 subjects were randomized at Week 0 to a single intravenous administration of ustekinumab of approximately 6 mg/kg, 130 mg (a lower dose than recommended), or placebo. Subjects enrolled in UC-1 had to have failed therapy with corticosteroids, immunomodulators or at least one biologic. A total of 51% had failed at least one biologic and 17% had failed both a TNF blocker and an integrin receptor blocker. Of the total population, 46% had failed corticosteroids or immunomodulators but were biologic-naïve and an additional 3% had previously received but had not failed a biologic. At induction baseline and throughout the trial, approximately 52% subjects were receiving oral corticosteroids, 28% subjects were receiving immunomodulators (AZA, 6-MP, or MTX) and 69% subjects were receiving aminosalicylates.
The primary endpoint was clinical remission at Week 8. Clinical remission with a definition of: Mayo stool frequency subscore of 0 or 1, Mayo rectal bleeding subscore of 0 (no rectal bleeding), and Mayo endoscopy subscore of 0 or 1 (Mayo endoscopy subscore of 0 defined as normal or inactive disease and Mayo subscore of 1 defined as presence of erythema, decreased vascular pattern and no friability) is provided in Table 15.
The secondary endpoints were clinical response, endoscopic improvement, and histologic-endoscopic mucosal improvement. Clinical response with a definition of (≥ 2 points and ≥ 30% decrease in modified Mayo score, defined as 3-component Mayo score without the Physician's Global Assessment, with either a decrease from baseline in the rectal bleeding subscore ≥ 1 or a rectal bleeding subscore of 0 or 1), endoscopic improvement with a definition of Mayo endoscopy subscore of 0 or 1, and histologic-endoscopic mucosal improvement with a definition of combined endoscopic improvement and histologic improvement of the colon tissue [neutrophil infiltration in < 5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue] are provided in Table 15.
In UC-1, a significantly greater proportion of subjects treated with ustekinumab (at the recommended dose of approximately 6 mg/kg dose) were in clinical remission and response and achieved endoscopic improvement and histologic-endoscopic mucosal improvement compared to placebo (see Table 15).
| Endpoint | Placebo N = 319 | Ustekinumab*
N = 322 | Treatment difference and 97.5% CI† | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
|
|
|||||
| Clinical Remission‡ | 22 | 7% | 62 | 19% | 12% (7%, 18%)§ |
| Bio-naïve¶ | 14/151 | 9% | 36/147 | 24% | |
| Prior biologic failure | 7/161 | 4% | 24/166 | 14% | |
| Endoscopic Improvement# | 40 | 13% | 80 | 25% | 12% (6%, 19%)§ |
| Bio-naïve¶ | 28/151 | 19% | 43/147 | 29% | |
| Prior biologic failure | 11/161 | 7% | 34/166 | 20% | |
| Clinical ResponseÞ | 99 | 31% | 186 | 58% | 27% (18%, 35%)§ |
| Bio-naïve¶ | 55/151 | 36% | 94/147 | 64% | |
| Prior biologic failure | 42/161 | 26% | 86/166 | 52% | |
| Histologic-Endoscopic Mucosal Improvementß | 26 | 8% | 54 | 17% | 9% (3%, 14%)§ |
| Bio-naïve¶ | 19/151 | 13% | 30/147 | 20% | |
| Prior biologic failure | 6/161 | 4% | 21/166 | 13% | |
The relationship of histologic-endoscopic mucosal improvement, as defined in UC-1, at Week 8 to disease progression and long-term outcomes was not evaluated during UC-1.
Trial UC-2
The maintenance trial (UC-2) evaluated 523 subjects who achieved clinical response 8 weeks following the intravenous administration of either induction dose of ustekinumab in UC-1. These subjects were randomized to receive a subcutaneous maintenance regimen of either 90 mg ustekinumab every 8 weeks, or every 12 weeks (a lower dose than recommended), or placebo for 44 weeks.
The primary endpoint was the proportion of subjects in clinical remission at Week 44. The secondary endpoints included the proportion of subjects maintaining clinical response at Week 44, the proportion of subjects with endoscopic improvement at Week 44, the proportion of subjects with corticosteroid-free clinical remission at Week 44, and the proportion of subjects maintaining clinical remission at Week 44 among subjects who achieved clinical remission 8 weeks after induction.
Results of the primary and secondary endpoints at Week 44 in subjects treated with ustekinumab at the recommended dosage (90 mg every 8 weeks) compared to the placebo are shown in Table 16.
| Endpoint | Placebo*
N = 175† | 90 mg ustekinumab every 8 weeks N = 176 | Treatment difference and 95% CI | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
|
|
|||||
| Clinical Remission‡ | 46 | 26% | 79 | 45% | 19% (9%, 28%)§ |
| Bio-naïve¶ | 30/84 | 36% | 39/79 | 49% | |
| Prior biologic failure | 16/88 | 18% | 37/91 | 41% | |
| Maintenance of Clinical Response at Week 44* | 84 | 48% | 130 | 74% | 26% (16%, 36%)§ |
| Bio-naïve¶ | 49/84 | 58% | 62/79 | 78% | |
| Prior biologic failure | 35/88 | 40% | 64/91 | 70% | |
| Endoscopic Improvement# | 47 | 27% | 83 | 47% | 20% (11%, 30%)§ |
| Bio-naïve¶ | 29/84 | 35% | 42/79 | 53% | |
| Prior biologic failure | 18/88 | 20% | 38/91 | 42% | |
| Corticosteroid-free Clinical RemissionÞ | 45 | 26% | 76 | 43% | 17% (8%, 27%)§ |
| Bio-naïve¶ | 30/84 | 36% | 38/79 | 48% | |
| Prior biologic failure | 15/88 | 17% | 35/91 | 38% | |
| Maintenance of Clinical Remission at Week 44 in subjects who achieved clinical remission 8 weeks after induction | 18/50 | 36% | 27/41 | 66% | 31% (12%, 50%)ß |
| Bio-naïve¶ | 12/27 | 44% | 14/20 | 70% | |
| Prior biologic failure | 6/23 | 26% | 12/18 | 67% | |
Other Endpoints
Week 16 Responders to Ustekinumab Induction
Subjects who were not in clinical response 8 weeks after induction with ustekinumab in UC-1 were not included in the primary efficacy analyses for trial UC-2; however, these subjects were eligible to receive a 90 mg subcutaneous injection of ustekinumab at Week 8. Of these subjects, 55/101 (54%) achieved clinical response eight weeks later (Week 16) and received ustekinumab 90 mg subcutaneously every 8 weeks during the UC-2 trial. At Week 44, there were 97/157 (62%) subjects who maintained clinical response and there were 51/157 (32%) who achieved clinical remission.
Histologic-Endoscopic Mucosal Improvement at Week 44
The proportion of subjects achieving histologic-endoscopic mucosal improvement during maintenance treatment in UC-2 was 75/172 (44%) among subjects on ustekinumab and 40/172 (23%) in subjects on placebo at Week 44. The relationship of histologic-endoscopic mucosal improvement, as defined in UC-2, at Week 44 to progression of disease or long-term outcomes was not evaluated in UC-2.
Endoscopic Normalization
Normalization of endoscopic appearance of the mucosa was defined as a Mayo endoscopic subscore of 0. At Week 8 in UC-1, endoscopic normalization was achieved in 25/322 (8%) of subjects treated with ustekinumab and 12/319 (4%) of subjects in the placebo group. At Week 44 of UC-2, endoscopic normalization was achieved in 51/176 (29%) of subjects treated with ustekinumab and in 32/175 (18%) of subjects in placebo group.
15 REFERENCES
- 1 Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 6.6.2 Regs Research Data, Nov 2009 Sub (1973–2007) – Linked To County Attributes - Total U.S., 1969–2007 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2010, based on the November 2009 submission.
16 HOW SUPPLIED/STORAGE AND HANDLING
WEZLANA (ustekinumab-auub) injection is a sterile, preservative-free, clear to opalescent and colorless to light yellow solution. It is supplied as individually packaged, single-dose prefilled syringes, single-dose vials, or single-dose prefilled ConfiPen autoinjectors.
For Subcutaneous Use
Prefilled Syringes
- 45 mg/0.5 mL (NDC: 55513-076-01, NDC: 72511-076-01)
- 90 mg/mL (NDC: 55513-089-01, NDC: 72511-089-01)
Each prefilled syringe is equipped with a 27 gauge fixed ½ inch needle, a needle safety guard, and a needle cover that does not contain dry natural rubber.
Single-dose Vial
- 45 mg/0.5 mL (NDC: 55513-055-01, NDC: 72511-055-01)
Single-dose Prefilled ConfiPen Autoinjector
- 45 mg/0.5 mL (NDC: 55513-102-01)
- 90 mg/mL (NDC: 55513-114-01)
The prefilled ConfiPen autoinjector is not made with natural rubber latex.
Storage and Stability
Store WEZLANA vials, prefilled syringes and prefilled ConfiPen autoinjectors refrigerated between 2°C to 8°C (36°F to 46°F). Keep the product in the original carton to protect from light until the time of use. Do not freeze. Do not shake.
If needed, individual prefilled syringe, 45 mg vial and prefilled ConfiPen autoinjector may be stored at room temperature up to 30°C (86°F) for a maximum single period of up to 30 days in the original carton to protect from light. Record the date when the prefilled syringe, the 45 mg vial or prefilled ConfiPen autoinjector is first removed from the refrigerator on the carton in the space provided. Once the prefilled syringe, the 45 mg vial or prefilled ConfiPen autoinjector has been stored at room temperature, do not return it to the refrigerator.
Discard the prefilled syringe, the 45 mg vial or prefilled ConfiPen autoinjector if not used within 30 days at room temperature storage. Do not use WEZLANA after the expiration date on the carton or on the prefilled syringe, on the 45 mg vial or on the prefilled ConfiPen autoinjector.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Infections
Inform patients that WEZLANA may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection [see Warnings and Precautions (5.1)].
Malignancies
Inform patients of the risk of developing malignancies while receiving WEZLANA [see Warnings and Precautions (5.4)].
Hypersensitivity Reactions
- Advise patients to seek immediate medical attention if they experience any signs or symptoms of serious hypersensitivity reactions and discontinue WEZLANA [see Warnings and Precautions (5.5)].
Posterior Reversible Encephalopathy Syndrome (PRES)
Inform patients to immediately contact their healthcare provider if they experience signs and symptoms of PRES (which may include headache, seizures, confusion, or visual disturbances) [see Warnings and Precautions (5.6)].
Immunizations
Inform patients that WEZLANA can interfere with the usual response to immunizations and that they should avoid live vaccines [see Warnings and Precautions (5.7)].
WEZLANA™ (ustekinumab-auub)
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, CA 91320-1799
U.S. License Number 1080
AMGEN, WEZLANA and ConfiPen are trademarks owned by Amgen Inc.
STELARA is a trademark of Johnson & Johnson.
© 2023, 2024 Amgen Inc. All rights reserved.
1xxxxxx-v2
| MEDICATION GUIDE WEZLANA™ (wez-LAH-nah) (ustekinumab-auub) injection, for subcutaneous or intravenous use |
|||
|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 12/2024 | ||
| What is the most important information I should know about WEZLANA?
WEZLANA is a medicine that affects your immune system. WEZLANA can increase your risk of having serious side effects, including: Serious infections. WEZLANA may lower the ability of your immune system to fight infections and may increase your risk of infections. Some people have serious infections while taking ustekinumab products, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses. Some people have to be hospitalized for treatment of their infection.
Before starting WEZLANA, tell your doctor if you:
|
|||
|
| ||
People who have a genetic problem where the body does not make any of the proteins interleukin 12 (IL-12) and interleukin 23 (IL-23) are at a higher risk for certain serious infections. These infections can spread throughout the body and cause death. People who take WEZLANA may also be more likely to get these infections. Cancers. WEZLANA may decrease the activity of your immune system and increase your risk for certain types of cancers. Tell your doctor if you have ever had any type of cancer. Some people who are receiving ustekinumab products and have risk factors for skin cancer have developed certain types of skin cancers. During your treatment with WEZLANA, tell your doctor if you develop any new skin growths. Posterior Reversible Encephalopathy Syndrome (PRES). PRES is a rare condition that affects the brain and can cause death. The cause of PRES is not known. If PRES is found early and treated, most people recover. Tell your doctor right away if you have any new or worsening medical problems including: |
|||
|
| ||
| What is WEZLANA?
WEZLANA is a prescription medicine used to treat:
|
|||
| Do not take WEZLANA if you are allergic to ustekinumab products or any of the ingredients in WEZLANA. See the end of this Medication Guide for a complete list of ingredients in WEZLANA. | |||
Before you receive WEZLANA, tell your doctor about all of your medical conditions, including if you:
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine. |
|||
How should I use WEZLANA?
Throw away any unused portion of WEZLANA. |
|||
| What should I avoid while using WEZLANA?
You should not receive a live vaccine while taking WEZLANA. See "Before you receive WEZLANA, tell your doctor about all of your medical conditions, including if you:" |
|||
| What are the possible side effects of WEZLANA?
WEZLANA may cause serious side effects, including:
|
|||
|
| ||
|
|||
|
| ||
| These are not all of the possible side effects of WEZLANA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to 1-800-77-AMGEN (1-800-772-6436). |
|||
How should I store WEZLANA?
Discard the prefilled syringe, the 45 mg vial or prefilled ConfiPen autoinjector if not used within 30 days at room temperature storage. Do not use WEZLANA after the expiration date on the carton or on the prefilled syringe, on the 45 mg vial or on the prefilled ConfiPen autoinjector. Keep WEZLANA and all medicines out of the reach of children. |
|||
| General information about the safe and effective use of WEZLANA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use WEZLANA for a condition for which it was not prescribed. Do not give WEZLANA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about WEZLANA that was written for health professionals. |
|||
| What are the ingredients in WEZLANA?
Active ingredient: ustekinumab-auub Inactive ingredients: Single-dose prefilled syringe for subcutaneous use contains histidine, histidine hydrochloride monohydrate, Polysorbate 80, and sucrose. Single-dose vial for subcutaneous use contains histidine, histidine hydrochloride monohydrate, Polysorbate 80 and sucrose. Single-dose prefilled ConfiPen autoinjector for subcutaneous use contains histidine, histidine hydrochloride monohydrate, Polysorbate 80, and sucrose. Single-dose vial for intravenous infusion contains edetate disodium, histidine, histidine hydrochloride monohydrate, methionine, Polysorbate 80, and sucrose. WEZLANA™(ustekinumab-auub) Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 U.S.A. US License Number 1080 © 2023–2024 Amgen Inc. All rights reserved. 1xxxxxx – v2 |
|||
INSTRUCTIONS FOR USE
WEZLANA™ (wez-LAH-nah)
(ustekinumab-auub)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject WEZLANA with a prefilled syringe.
The medicine in the WEZLANA prefilled syringe is for injection under-the-skin (subcutaneous injection). See the WEZLANA Medication Guide for more information about WEZLANA.
| Getting to Know Your Prefilled Syringe |
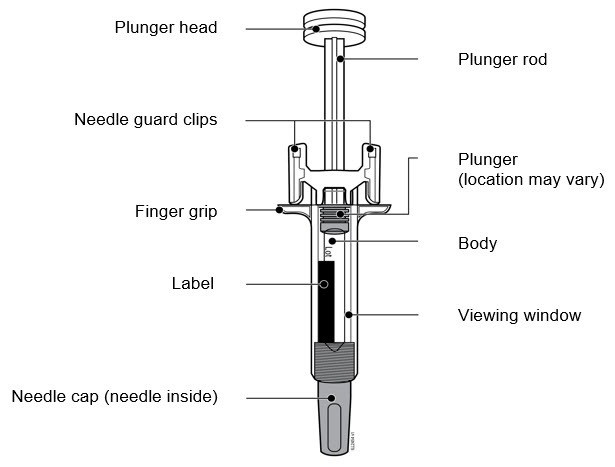 |
Step 1. Important Information You Need to Know Before Injecting WEZLANA
Dosing:
- WEZLANA comes in two different doses: 45 mg and 90 mg. Check your prescription to make sure you have the correct dose.
- If your dose is 45 mg, you will receive one 45 mg prefilled syringe.
- If your dose is 90 mg, you will receive either one 90 mg prefilled syringe or two 45 mg prefilled syringes.
- If you receive two 45 mg prefilled syringes, you will need to give yourself two injections, one right after the other.
- The look of the prefilled syringe will be different for each dose. The amount of medicine in the syringe will also be different for each dose.
- The 45 mg dose will have a smaller amount of medicine and the 90 mg dose will have a larger amount of medicine. Check the figures below to see what your dose looks like in the syringe.
 45 mg/0.5 mL |  90 mg/mL |
Using your WEZLANA prefilled syringe:
- It is important that you do not try to give the injection until you have fully read and understood this Instructions for Use.
- If your doctor decides that you or a caregiver can give your WEZLANA injection at home, you should receive training on the right way to prepare and inject WEZLANA. Do not try to inject WEZLANA yourself until you have been shown the right way to give the injections by your doctor or nurse.
- Children 12 years of age and older with psoriasis who weigh 132 pounds or more may use a prefilled syringe under supervision of a parent or caregiver.
- Do not use the syringe if the carton is damaged or seal is broken.
- Do not use the syringe after the expiration date on the label.
- Do not shake the syringe.
- Do not remove the needle cap from the syringe until you are ready to inject.
- Do not use the syringe if it has been frozen.
- Do not use the syringe if it has been dropped on a hard surface. Part of the syringe may be broken even if you cannot see the break. Use a new syringe and call 1-800-77-AMGEN (1-800-772-6436).
- The syringe is not made with natural rubber latex.
| Important: Keep the syringe and sharps disposal container out of the sight and reach of children. |
Frequently asked questions:
For additional information and answers to frequently asked questions, visit www.WEZLANA.com.
Where to get help:
If you want more information or help using WEZLANA:
- Contact your doctor or nurse
- Visit www.WEZLANA.com, or
- Call 1-800-77-AMGEN (1-800-772-6436).
Step 2. Storing and Preparing to Inject WEZLANA
2a Refrigerate the syringe carton until you are ready to use it.
- Keep the syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the syringe in the original carton to protect it from light or physical damage until you are ready to use it.
- Do not freeze the syringe.
- Do not store the syringe in extreme heat or cold. For example, avoid storing in your vehicle`s glove box or trunk.
| Important: Keep the syringe out of the sight and reach of children. |
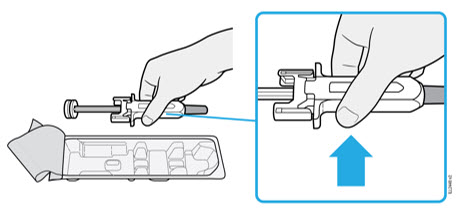
2b Grasp the syringe by the body and remove it from the carton. To prevent early activation of the needle safety guard, do not touch the NEEDLE GUARD CLIPS at any time during use.
- Do not grab the plunger rod, finger grip or the needle cap.
- Do not grab the needle guard clips.
- Remove the number of syringes you need for your injection.
- Put any unused syringes back into refrigerator.
WAIT
2c Wait 30 minutes for the syringe to reach room temperature.
- Let the syringe warm up naturally.
- Do not heat with hot water, a microwave, or direct sunlight.
- Do not shake the syringe at any time.
- Using the syringe at room temperature allows for a more comfortable injection.

2d You may also keep WEZLANA at room temperature for up to 30 days, if needed.
- Keep it at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not use WEZLANA if the syringe has been stored above 86°F (30°C).
- Do not put WEZLANA back in refrigerator after it has been stored at room temperature.
- Record the date you removed WEZLANA from the refrigerator and use within 30 days.
| Important: Place the syringe in a sharps disposal container if it has reached room temperature and has not been used within 30 days. |
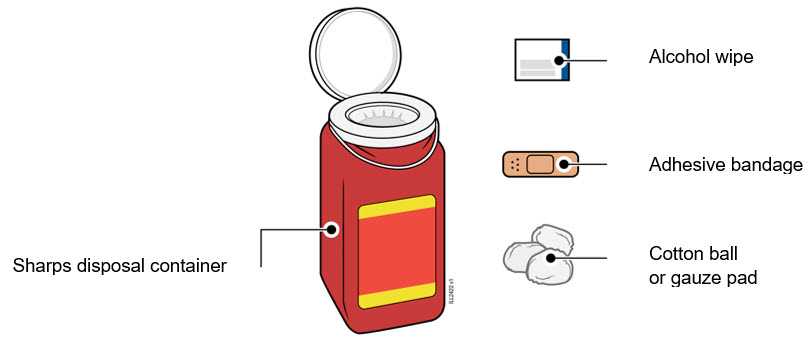
2e Gather and place the following items for your injection on a clean, flat, and well-lit surface:
- WEZLANA syringe (room temperature)
- Sharps disposal container (see Step 5. Disposing of WEZLANA and Checking the Injection Site)
- Alcohol wipe
- Adhesive bandage
- Cotton ball or gauze pad
Step 3. Getting Ready for Your Injection
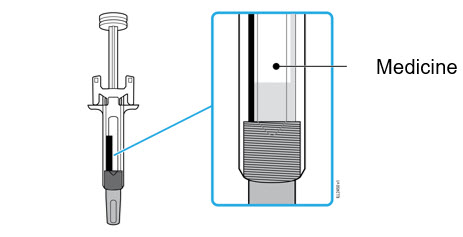
3a Inspect the medicine through the viewing window. It should be clear to white like an opal and colorless to light yellow.
- It is okay to see air bubbles.
- Do not use WEZLANA if the medicine is frozen, cloudy, discolored, or has large particles.
| Important: If the medicine is cloudy, discolored or has flakes or if the syringe is damaged or expired, call 1-800-77-AMGEN (1-800-772-6436). |
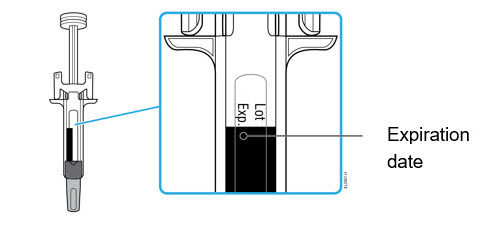
3b Check the expiration (Exp.) date and inspect the syringe for damage.
- Do not use if the expiration date has passed.
-
Do not use the syringe if:
- the needle cap is missing or loose
- it has cracks or broken parts
- it has been dropped on a hard surface or dropped without the needle cap in place
- Make sure you have the right medicine and dose.
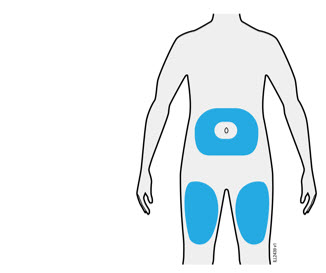
3c Choose 1 of these injection locations.
- Choose an injection site around your stomach area (abdomen) or upper legs (thighs).
- If a caregiver is giving you the injection, the outer upper area of the upper arms, or the buttocks may also be used.
- Choose a different location (site) for each injection.
- The areas in blue are the recommended injection locations.
| Important: Avoid areas where the skin is tender, bruised, red, or hard. |
3d Wash your hands thoroughly with soap and water.
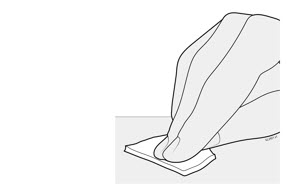
3e Clean the injection site with alcohol wipe.
- Let your skin dry on its own.
- Do not touch the clean area again before injecting.
Step 4. Injecting WEZLANA
| Important: Only remove the needle cap when you can inject WEZLANA right away (within 5 minutes) because the medicine can dry out. |
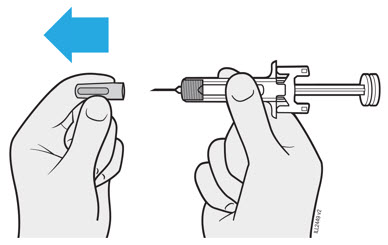
4a Pull the needle cap straight off while holding the syringe body.
- Do not hold the plunger or plunger head while removing the needle cap.
- Do not twist or bend the needle cap.
- Never put the needle cap back on. It may damage the needle.
- Do not let anything touch the needle after the cap is removed.
- Do not place the syringe on any surface after the cap is removed.
- Do not try to push air bubbles out. It is okay to see air bubbles.
- A drop of medicine at the needle tip is normal.
PINCH
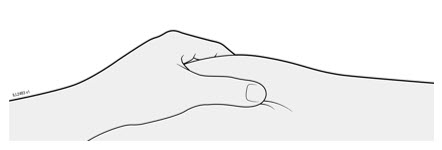
4b Pinch the skin around injection site before injection.
- Gently pinch the skin with one hand between the thumb and index finger to create a bump for the injection.
- If possible, the bump should be about 2 inches wide.
Important: Continue to pinch the skin until the injection is complete.
INSERT
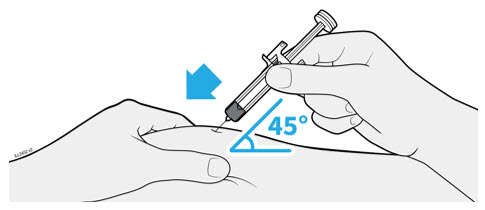
4c Insert the needle into the pinched skin.
- Insert the needle into the pinched skin at about a 45-degree angle.
-
Do not place your finger on the plunger rod while inserting the needle, as this may result in lost medicine.
INJECT
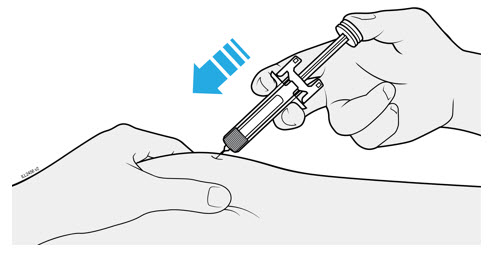
4d Slowly press the plunger head all the way down with your thumb until it is completely between the needle guard clips. Hold the finger grips with your fingers as shown in the figure.
- Do not pull back on the plunger rod at any time.
-
Do not remove the syringe until all medicine has been injected.
LIFT
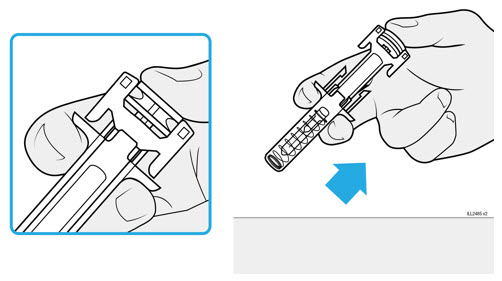
4e Keep pressure on the plunger head with your thumb and remove the needle from your skin.
- Let go of the skin after the needle is removed.
- Slowly take your thumb off the plunger head. This will let the empty syringe move up until the entire needle is automatically covered by the needle guard.
If a second injection is required:
4f Repeat steps 2a-4e if a second injection is required.
- Check your prescription for your dose.
- If your dose is 90 mg, you will receive either one 90 mg prefilled syringe or two 45 mg prefilled syringes.
- If you receive two 45 mg prefilled syringes for a 90 mg dose, you will need to give yourself a second injection right after the first injection.
- Repeat Steps 2a–4e for the second injection using a new WEZLANA syringe.
- Choose a different site for the second injection.
Step 5. Disposing of WEZLANA and Checking the Injection Site
| Important: Never put the needle cap back on the syringe. |
5a Place the used syringe and needle cap in an FDA-cleared sharps disposal container right away after use.
- Do not reuse the syringe.
| Important: Do not throw away (dispose of) the syringe in your household trash. |
5b Check injection site.
- Do not rub the injection site.
- If there is blood, press a cotton ball or gauze pad on your injection site.
- Apply an adhesive bandage if necessary
Additional information about your sharps disposal container
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
Disposing of sharps containers:
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container.
There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
Do not recycle your used sharps disposal container.
For more information or help call 1-800-77-AMGEN (1-800-772-6436).
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
US License Number 1080
© 2023 Amgen Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 10/2023
INSTRUCTIONS FOR USE
WEZLANA™ (wez-LAH-nah)
(ustekinumab-auub)
injection, for subcutaneous use
single-dose vial
Instructions for injecting WEZLANA from a vial.
Read this Instructions for Use before you start using WEZLANA. Your doctor or nurse should show you how to prepare, measure your dose, and give your injection of WEZLANA the right way.
If you cannot give yourself the injection:
- ask your doctor or nurse to help you, or
- ask someone who has been trained by a doctor or nurse to give your injections.
Do not try to inject WEZLANA yourself until you have been shown how to inject WEZLANA by your doctor, nurse or health professional.
Important information:
- Before you start, check the carton to make sure that it is the right dose. You will have either 45 mg or 90 mg as prescribed by your doctor.
- If your dose is 45 mg or less, you will receive one 45 mg vial.
- If your dose is 90 mg, you will receive two 45 mg vials and you will need to give yourself two injections, one right after the other.
- Children 12 years of age and older weighing less than 132 pounds require a dose lower than 45 mg.
- Check the expiration date on the vial and carton. If the expiration date has passed, do not use it. If the expiration date has passed, call your doctor or pharmacist, or call 1-800-77-AMGEN (1-800-772-6436) for help.
- Check the vial for any particles or discoloration. Your vial should look clear to white like an opal and colorless to light yellow.
- Do not use if it is frozen, discolored, cloudy or has large particles. Get a new vial.
- Do not shake the vial at any time. Shaking your vial may damage your WEZLANA medicine. If your vial has been shaken, do not use it. Get a new vial.
- Keep the vial in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original container to protect from light until you are ready to use it.
- The WEZLANA vial can also be stored at room temperature up to 86°F (30°C) for up to 30 days in the original carton. Write the date that you removed WEZLANA from the refrigerator on the carton in the space provided.
- Do not use WEZLANA if it has been stored above 86°F (30°C).
- Do not put WEZLANA back in the refrigerator after it has been stored at room temperature.
- Do not use a WEZLANA vial more than one time, even if there is medicine left in the vial. After the rubber stopper is punctured, WEZLANA can become contaminated by harmful bacteria which could cause an infection if re-used. Therefore, throw away any unused WEZLANA after you give your injection.
- Safely throw away (dispose of) WEZLANA vials after use.
- Do not re-use syringes or needles. See "Step 6: Dispose of the needles and syringes."
- To avoid needle-stick injuries, do not recap needles.
Gather supplies you will need to prepare WEZLANA and to give your injection. (See Figure A)
You will need:
- a syringe with the needle attached, you will need a prescription from your healthcare provider to get syringes with the needles attached from your pharmacy
- alcohol wipes
- cotton balls or gauze pads
- adhesive bandage
- your prescribed dose of WEZLANA
- FDA-cleared sharps disposal container. See "Step 6: Dispose of the needles and syringes."
Step 1. Prepare the injection.
- Choose a well-lit, clean, flat work surface.
- Wash your hands well with soap and warm water.
Step 2. Prepare your injection site.
* Areas in blue are recommended injection sites.
- Choose an injection site around your stomach area (abdomen) or upper legs (thighs).
- If a caregiver is giving you the injection, the outer area of the upper arms, or the buttocks may also be used. (See Figure B)
- Use a different injection site for each injection. Do not give an injection in an area of the skin that is tender, bruised, red or hard.
- Clean the skin with an alcohol wipe where you plan to give your injection.
- Do not touch this area again before giving the injection. Let your skin dry before injecting.
- Do not fan or blow on the clean area.
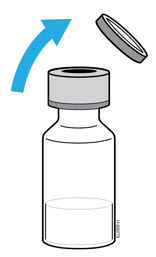
3a
- Remove the cap from the top of the vial. Throw away the cap but do not remove the rubber stopper. (See Figure C)
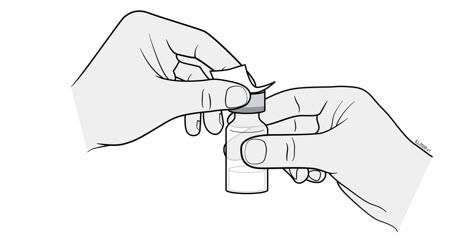
3b
- Clean the rubber stopper with an alcohol wipe. (See Figure D)
- Do not touch the rubber stopper after you clean it.
- Put the vial on a flat surface.
Step 4. Prepare the syringe.
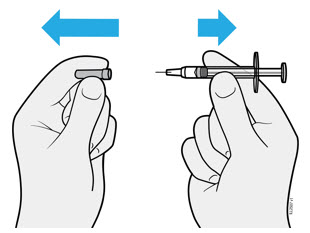
4a
- Pick up the syringe with the needle attached.
- Remove the cap that covers the needle. (See Figure E)
- Throw the needle cap away. Do not touch the needle or allow the needle to touch anything.
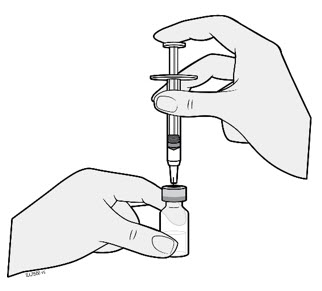
4b
- Carefully pull back on the plunger to the line that matches the dose prescribed by your doctor.
- Hold the vial between your thumb and index (pointer) finger.
- Use your other hand to push the syringe needle through the center of the rubber stopper. (See Figure F)
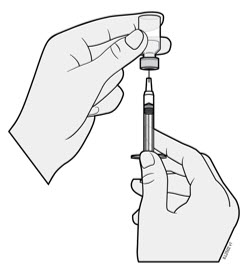
4c
- Push down on the plunger until all of the air has gone from the syringe into the vial.
- Turn the vial and the syringe upside down. (See Figure G)
- Hold the WEZLANA vial with one hand.
- It is important that the needle is always in the liquid in order to prevent air bubbles forming in the syringe.
- Pull back on the syringe plunger with your other hand.
- Fill the syringe until the black tip of the plunger lines up with the mark that matches your prescribed dose.
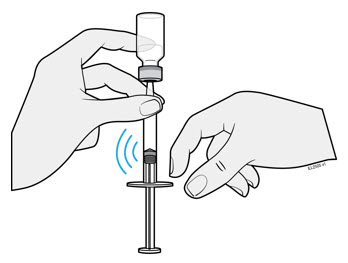
4d
- Do not remove the needle from the vial. Hold the syringe with the needle pointing up to see if it has any air bubbles inside.
- If there are air bubbles, gently tap the side of the syringe until the air bubbles rise to the top. (See Figure H)
- Slowly press the plunger up until all of the air bubbles are out of the syringe (but none of the liquid is out).
- Remove the syringe from the vial. Do not lay the syringe down or allow the needle to touch anything.
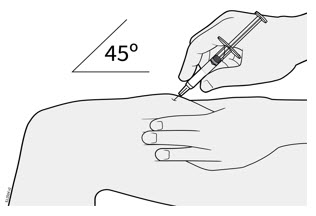
5a
- Hold the barrel of the syringe in one hand, between the thumb and index fingers.
- Do not pull back on the plunger at any time.
- Use the other hand to gently pinch the cleaned area of skin. Hold firmly.
- Use a quick, dart-like motion to insert the needle into the pinched skin at about a 45-degree angle. (See Figure I)
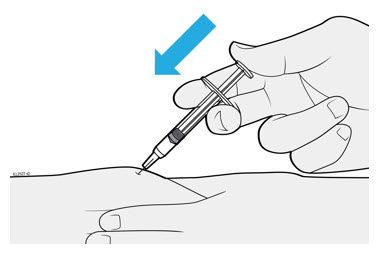
5b
- Push the plunger with your thumb as far as it will go to inject all of the liquid. Push it slowly and evenly, keeping the skin gently pinched. (See Figure J)
- When the syringe is empty, pull the needle out of your skin and let go of the skin.
- When the needle is pulled out of your skin, there may be a little bleeding at the injection site. This is normal. You can press a cotton ball or gauze pad to the injection site if needed. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if necessary.
If your dose is 90 mg, you will receive two 45 mg vials and you will need to give yourself a second injection right after the first. Repeat Steps 1-5 using a new syringe. Choose a different site for the second injection.
Step 6. Dispose of the needles and syringes.
- Do not re-use a syringe or needle.
- To avoid needle-stick injuries, do not recap a needle.
- Put your needles and syringes in an FDA-cleared sharps disposal container right away after use.
Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and,
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how you should throw away syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps container in your household trash unless your community guidelines permit this. Do not recycle your sharps disposal container.
- Throw away the vial into the container where you put the syringes and needles.
- If you have any questions, talk to your doctor or pharmacist.
Keep WEZLANA and all medicines out of the reach of children.
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
US License Number 1080
© 2023 Amgen Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued 10/2023
PRINCIPAL DISPLAY PANEL - 45 mg/0.5 mL Syringe Carton
NDC: 72511-076-01
WEZLANA™
(ustekinumab-auub)
Injection
45
mg/0.5 mL
45 mg/0.5 mL
For Subcutaneous Use Only
ATTENTION: Dispense the enclosed
Medication Guide to each patient.
Sterile Solution - No Preservative
Store refrigerated at 2° to 8°C (36° to 46°F).
Do not shake. Do not freeze.
Keep out of reach of children.
Keep in carton to protect from light.
!
CAUTION: See full prescribing information and
Instructions for Use.
Contains 1 Single-dose Prefilled Syringe
Discard unused portion
Do not re-use
Rx Only
AMGEN®

PRINCIPAL DISPLAY PANEL - 90 mg/mL Syringe Carton
NDC: 72511-089-01
WEZLANA™
(ustekinumab-auub)
Injection
90
mg/mL
90 mg/mL
For Subcutaneous Use Only
ATTENTION: Dispense the enclosed
Medication Guide to each patient.
Sterile Solution - No Preservative
Store refrigerated at 2° to 8°C (36° to 46°F).
Do not shake. Do not freeze.
Keep out of reach of children.
Keep in carton to protect from light.
!
CAUTION: See full prescribing information
and Instructions for Use.
Contains 1 Single-dose Prefilled Syringe
Discard unused portion
Do not re-use
Rx Only
AMGEN®

PRINCIPAL DISPLAY PANEL - 45 mg/0.5 mL Vial Carton
NDC: 72511-055-01
WEZLANA™
(ustekinumab-auub)
Injection
45
mg/0.5 mL
45 mg/0.5 mL
For Subcutaneous Use Only
ATTENTION: Dispense the enclosed
Medication Guide to each patient.
Keep out of reach of children.
Store refrigerated at 2°C to
8°C (36°F to 46°F) in the
original carton to protect
from light. Do not shake. Do
not freeze.
Contains 1 Single-dose Vial
Discard unused portion
Sterile Solution - No
Preservative
Rx Only
AMGEN®
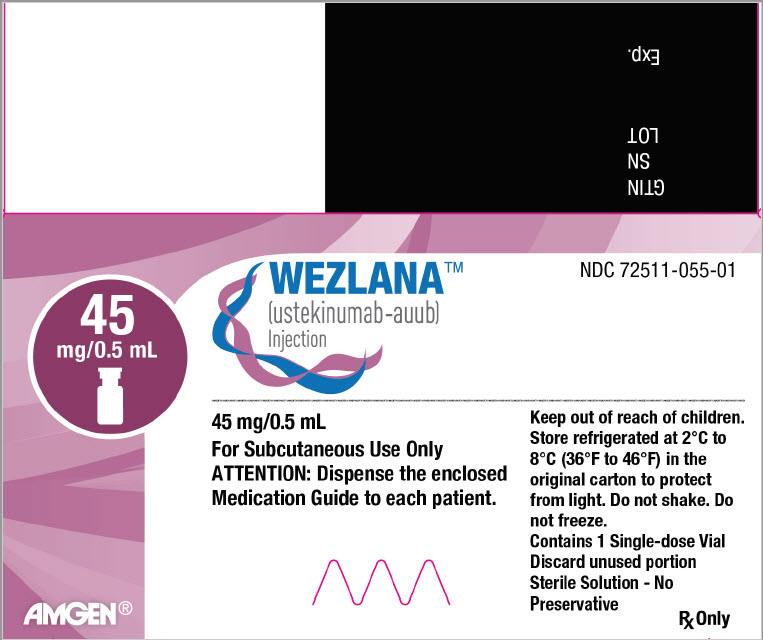
| WEZLANA
ustekinumab-auub injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| WEZLANA
ustekinumab-auub injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| WEZLANA
ustekinumab-auub injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amgen USA Inc. (962075045) |
| Registrant - Immunex Rhode Island Corporation (968084785) |
Trademark Results [WEZLANA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WEZLANA 97826509 not registered Live/Pending |
Amgen Inc. 2023-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.