ALDURAZYME- laronidase injection, solution, concentrate
ALDURAZYME by
Drug Labeling and Warnings
ALDURAZYME by is a Prescription medication manufactured, distributed, or labeled by Genzyme Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALDURAZYME safely and effectively. See full prescribing information for ALDURAZYME.
ALDURAZYME (laronidase) injection, for intravenous use
Initial U.S. Approval: 2003WARNING: RISK OF ANAPHYLAXIS
See full prescribing information for complete boxed warning.
- Life-threatening anaphylactic reactions have been observed in some patients during ALDURAZYME infusions. (5.1)
- Appropriate medical support should be readily available when ALDURAZYME is administered. (5.1)
- Patients with compromised respiratory function or acute respiratory disease may be at risk of serious acute exacerbation of their respiratory compromise due to infusion reactions and require additional monitoring. (5.2, 5.3)
INDICATIONS AND USAGE
ALDURAZYME is a hydrolytic lysosomal glycosaminoglycan (GAG)-specific enzyme indicated for adult and pediatric patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I (MPS I) and for patients with the Scheie form who have moderate to severe symptoms. (1)
Limitations of Use:
DOSAGE AND ADMINISTRATION
The recommended dosage is 0.58 mg/kg of body weight administered once weekly as an intravenous infusion (2).
DOSAGE FORMS AND STRENGTHS
Injection: 2.9 mg/5 mL (0.58 mg/mL) of laronidase in a single-dose vial (3).
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Anaphylaxis and Hypersensitivity Reactions: Life-threatening anaphylactic reactions have been observed in some patients during ALDURAZYME infusion and up to 3 hours after infusion. Appropriate medical support and monitoring measures should be readily available when ALDURAZYME is administered. If anaphylactic or other severe hypersensitivity reactions occur, immediately discontinue the infusion and initiate appropriate treatment, which may include ventilatory support, treatment with inhaled beta-adrenergic agonists, epinephrine, and IV corticosteroids. (5.1)
- Risk of Acute Respiratory Complications: Patients with acute febrile or respiratory illness at the time of ALDURAZYME infusion may be at greater risk for infusion reactions. Consider delaying ALDURAZYME infusion. Sleep apnea is common in MPS I patients. Evaluation of airway patency should be considered prior to initiation of treatment with ALDURAZYME. Appropriate respiratory support should be available during infusion. (5.2)
- Risk of Acute Cardiorespiratory Failure: Caution should be exercised when administering ALDURAZYME to patients susceptible to fluid overload. Consider a decreased total infusion volume and infusion rate when administering ALDURAZYME to these patients. Appropriate medical monitoring and support measures should be available during infusion. (2.2, 5.3)
- Infusion Reactions: Pretreatment is recommended prior to the infusion to reduce the risk of infusion reactions and may include antihistamines, antipyretics, or both. If infusion reactions occur, decreasing the infusion rate, temporarily stopping the infusion, or administering additional antipyretics and/or antihistamines may ameliorate the symptoms. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (≥10%) in patients:
-
6 months of age and older are: infusion reactions (pyrexia, chills, blood pressure increased, tachycardia, and oxygen saturation decreased). (6.1)
-
6 years and older are: rash, upper respiratory tract infection, injection site reaction, hyperreflexia, paresthesia, flushing, and poor venous access. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact: Genzyme at 1-800-745-4447, or FDA at 1-800-FDA-1088 or go to www.fda.gov/medwatch.See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF ANAPHYLAXIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Hypersensitivity Reactions
5.2 Acute Respiratory Complications Associated with Administration
5.3 Risk of Acute Cardiorespiratory Failure
5.4 Infusion Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Studies in Patients 6 Years and Older
14.2 Clinical Studies in Patients 6 Years and Younger
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF ANAPHYLAXIS
- Life-threatening anaphylactic reactions have been observed in some patients during ALDURAZYME® infusions [see Warnings and Precautions (5.1)].
- Appropriate medical support should be readily available when ALDURAZYME is administered [see Warnings and Precautions (5.1)].
- Patients with compromised respiratory function or acute respiratory disease may be at risk of serious acute exacerbation of their respiratory compromise due to infusion reactions and require additional monitoring [see Warnings and Precautions (5.2, 5.3)].
-
1 INDICATIONS AND USAGE
ALDURAZYME® is indicated for adult and pediatric patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I (MPS I) and for patients with the Scheie form who have moderate to severe symptoms.
Limitations of Use:
-
The risks and benefits of treating mildly affected patients with the Scheie form have not been established.
-
ALDURAZYME has not been evaluated for effects on the central nervous system manifestations of the disorder.
-
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dosage regimen of ALDURAZYME is 0.58 mg/kg of body weight administered once weekly as an intravenous infusion. Pretreatment is recommended 60 minutes prior to the start of the infusion and may include antihistamines, antipyretics, or both [see Warnings and Precautions (5)].
Each vial of ALDURAZYME provides 2.9 milligrams (mg) of laronidase in 5.0 milliliters (mL) of solution and is intended for single dose only. Do not use the vial more than one time. The concentrated solution for infusion must be diluted with 0.9% Sodium Chloride Injection, USP, to a final volume of 100 mL or 250 mL, using aseptic techniques. The final volume of the infusion is determined by the patient’s body weight. Patients with a body weight of 20 kg or less should receive a total volume of 100 mL. Patients with a body weight greater than 20 kg should receive a total volume of 250 mL [see Dosage and Administration (2.2)]. For patients with underlying cardiac or respiratory compromise and weighing up to 30 kg, physicians may consider diluting ALDURAZYME in a volume of 100 mL and administering at a decreased infusion rate [see Dosage and Administration (2.2), Warnings and Precautions (5.3), Adverse Reactions (6.3)].
2.2 Instructions for Use
Prepare and use ALDURAZYME according to the following steps. Use aseptic techniques. Prepare ALDURAZYME using low-protein-binding containers and administer with a low-protein-binding infusion set equipped with an in-line, low-protein-binding 0.2 micron filter. There is no information on the compatibility of diluted ALDURAZYME with glass containers.
-
Determine the number of vials to be diluted based on the patient's weight and the recommended dose of 0.58 mg/kg, using the following equation:
Patient's weight (kg) × 1 mL/kg of ALDURAZYME = Total number of mL of ALDURAZYME
Total number of mL of ALDURAZYME ¸ 5 mL per Vial = Total number of Vials. -
Round up to the next whole vial. Remove the required number of vials from the refrigerator to allow them to reach room temperature. Do not heat or microwave vials.
-
Before withdrawing the ALDURAZYME from the vial, visually inspect each vial for particulate matter and discoloration. The ALDURAZYME solution should be clear to slightly opalescent and colorless to pale yellow. Some translucency may be present in the solution. Do not use if the solution is discolored or if there is particulate matter in the solution.
-
Withdraw and discard a volume of the 0.9% Sodium Chloride Injection, USP from the infusion bag, equal to the volume of ALDURAZYME concentrate to be added.
-
Slowly withdraw the calculated volume of ALDURAZYME from the appropriate number of vials using caution to avoid excessive agitation. Do not use a filter needle, as this may cause agitation. Agitation may denature ALDURAZYME, rendering it biologically inactive.
-
Slowly add the ALDURAZYME solution to the 0.9% Sodium Chloride Injection, USP using care to avoid agitation of the solutions. Do not use a filter needle.
-
Gently rotate the infusion bag to ensure proper distribution of ALDURAZYME. Do not shake the solution.
-
The entire infusion volume (100 mL for patients weighing 20 kg or less and 250 mL for patients weighing greater than 20 kg) should be delivered over approximately 3 to 4 hours. The initial infusion rate of 10 µg/kg/hr may be incrementally increased every 15 minutes during the first hour, as tolerated, until a maximum infusion rate of 200 µg/kg/hr is reached. The maximum rate is then maintained for the remainder of the infusion (2-3 hours), as outlined in Tables 1 and 2.
-
Administer the diluted ALDURAZYME solution to patients using a low-protein-binding infusion set equipped with a low-protein-binding 0.2 µm in-line filter.
Table 1: Incremental Rates for 100 mL ALDURAZYME® Infusion (For use with Patients Weighing 20 kg or Less) Infusion Rate Criteria for Increasing Infusion Rate 2 mL/hour x 15 minutes (10 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 4 mL/hour x 15 minutes (20 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 8 mL/hour x 15 minutes (50 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 16 mL/hour x 15 minutes (100 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 32 mL/hour x ~3 hours (200 mcg/kg/hr) For the remainder of the infusion. Table 2: Incremental Rates for 250 mL ALDURAZYME® Infusion (For use with Patients Weighing Greater than 20 kg) Infusion Rate Criteria for Increasing Infusion Rate 5 mL/hour x 15 minutes (10 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 10 mL/hour x 15 minutes (20 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 20 mL/hour x 15 minutes (50 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 40 mL/hour x 15 minutes (100 mcg/kg/hr) Obtain vital signs, if stable then increase the rate to... 80 mL/hour x approximately 3 hours (200 mcg/kg/hr) For the remainder of the infusion. ALDURAZYME does not contain any preservatives; therefore, after dilution with saline, the infusion bags should be used immediately. If immediate use is not possible, the diluted solution should be stored refrigerated at 2°C to 8°C (36°F to 46°F) for up to 36 hours. Other than during infusion, room temperature storage of diluted solution is not recommended. Any unused product or waste material should be discarded and disposed of in accordance with local requirements.
ALDURAZYME must not be administered with other medicinal products in the same infusion. The compatibility of ALDURAZYME in solution with other products has not been evaluated.
-
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Hypersensitivity Reactions
Anaphylaxis and serious hypersensitivity reactions have been observed in patients during or up to 3 hours after ALDURAZYME infusions. Some of these reactions were life-threatening and included respiratory failure, respiratory distress, stridor, tachypnea, bronchospasm, obstructive airways disorder, hypoxia, hypotension, bradycardia, and urticaria. If anaphylactic or other serious hypersensitivity reactions occur, immediately discontinue the infusion of ALDURAZYME and initiate appropriate medical treatment. Caution should be exercised if epinephrine is being considered for use in patients with MPS I due to the increased prevalence of coronary artery disease in these patients. Interventions have included resuscitation, mechanical ventilatory support, emergency tracheotomy, hospitalization, and treatment with inhaled beta-adrenergic agonists, epinephrine, and intravenous corticosteroids [see Adverse Reactions (6)].
In clinical studies and postmarketing safety experience with ALDURAZYME, approximately 1% of patients experienced severe or serious hypersensitivity reactions. In patients with MPS I, pre-existing upper airway obstruction may have contributed to the severity of some reactions. Due to the potential for severe hypersensitivity reactions, appropriate medical support should be readily available when ALDURAZYME is administered. Because of the potential for recurrent reactions, some patients who experience initial severe reactions may require prolonged observation.
The risks and benefits of re-administering ALDURAZYME following an anaphylactic or severe hypersensitivity reaction should be considered. Extreme care should be exercised, with appropriate resuscitation measures available, if the decision is made to re-administer the product.
5.2 Acute Respiratory Complications Associated with Administration
Patients with an acute febrile or respiratory illness at the time of ALDURAZYME infusion may be at greater risk for infusion reactions. Careful consideration should be given to the patient’s clinical status prior to administration of ALDURAZYME and consider delaying ALDURAZYME infusion. One patient with acute bronchitis and hypoxia experienced increased tachypnea during the first ALDURAZYME infusion that resolved without intervention. The patient’s respiratory symptoms returned within 30 minutes of completing the infusion and responded to bronchodilator therapy. Approximately 6 hours after the infusion, the patient experienced coughing, then respiratory arrest, and died.
Sleep apnea is common in MPS I patients. Evaluation of airway patency should be considered prior to initiation of treatment with ALDURAZYME. Patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep should have these treatments readily available during infusion in the event of an infusion reaction, or extreme drowsiness/sleep induced by antihistamine use.
5.3 Risk of Acute Cardiorespiratory Failure
Caution should be exercised when administering ALDURAZYME to patients susceptible to fluid overload, or patients with acute underlying respiratory illness or compromised cardiac and/or respiratory function for whom fluid restriction is indicated. These patients may be at risk of serious exacerbation of their cardiac or respiratory status during infusions. Appropriate medical support and monitoring measures should be readily available during ALDURAZYME infusion, and some patients may require prolonged observation times that should be based on the individual needs of the patient [see Adverse Reactions (6.3)].
5.4 Infusion Reactions
Because of the potential for infusion reactions, patients should receive antipyretics and/or antihistamines prior to infusion. If an infusion reaction occurs, regardless of pretreatment, decreasing the infusion rate, temporarily stopping the infusion, or administering additional antipyretics and/or antihistamines may ameliorate the symptoms [see Adverse Reactions (6.1, 6.3)].
-
6 ADVERSE REACTIONS
Serious and or clinically significant adverse reactions described elsewhere in labeling include:
-
Anaphylaxis and Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
-
Acute Respiratory Complications Associated with Administration [see Warnings and Precautions (5.2)]
-
Risk of Acute Cardiorespiratory Failure [see Warnings and Precautions (5.3)]
-
Infusion Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most serious adverse reactions reported with ALDURAZYME treatment during clinical trials were anaphylactic and hypersensitivity reactions. Most adverse reactions reported in clinical trials were considered disease-related and unrelated to study drug. The most common adverse reactions were infusion reactions. The frequency of infusion reactions decreased over time with continued use of ALDURAZYME, and the majority of reactions were classified as being mild to moderate in severity. Most infusion reactions requiring intervention were ameliorated with slowing of the infusion rate, temporarily stopping the infusion, with or without administering additional treatments including antihistamines, antipyretics, or both.
Clinical Trials in Patients 6 Years and Older
A 26-week, double-blind, placebo-controlled clinical study (Study 1) of ALDURAZYME was conducted in 45 patients with MPS I, ages 6 to 43 years old, gender evenly distributed (N=23 females and 22 males). Of these 45 patients, 1 was clinically assessed as having Hurler form, 37 Hurler-Scheie, and 7 Scheie. Patients were randomized to receive either 0.58 mg/kg intravenously of ALDURAZYME per week for 26 weeks or placebo. All patients were treated with antipyretics and antihistamines prior to the infusions. Infusion reactions were reported in 32% (7 of 22) of ALDURAZYME-treated patients. The most commonly reported infusion reactions regardless of treatment group were flushing, pyrexia, headache, and rash. Flushing occurred in 5 patients (23%) receiving ALDURAZYME; the other reactions were less frequent. Less common infusion reactions included angioedema (including face edema), hypotension, paresthesia, feeling hot, hyperhidrosis, tachycardia, vomiting, back pain, and cough. Other reported adverse reactions included bronchospasm, dyspnea, urticaria and pruritus.
Table 3 enumerates adverse reactions and selected laboratory abnormalities that occurred during the placebo-controlled study (Study 1) that were reported in at least 2 patients more in the ALDURAZYME group than in the placebo group.
Table 3: Summary of Adverse Reactions that Occurred in 2 Patients More in the ALDURAZYME® Group than in the Placebo Group in the 26-Week Placebo-controlled Study (Study 1)
MedDRA System Organ Class (SOC)
MedDRA Preferred Term(N=22)
ALDURAZYME
n (%)(N=23)
Placebo
n (%)Blood and lymphatic system disorders Thrombocytopenia 2 (9) 0 Eye disorders Corneal opacity 2 (9) 0 General disorders and administration site conditions Chest pain 2 (9) 0 Face edema 2 (9) 0 Gravitational edema 2 (9) 0 Injection site pain 2 (9) 0 Injection site reaction 4 (18) 2 (9) Hepatobiliary disorders Hyperbilirubinemia 2 (9) 0 Infections and infestations Abscess 2 (9) 0 Upper respiratory tract infection 7 (32) 4 (17) Nervous system disorders Hyperreflexia 3 (14) 0 Paresthesia 3 (14) 1 (4) Skin and subcutaneous tissue disorders Rash 8 (36) 5 (22) Vascular disorders Hypotension 2 (9) 0 Poor venous access 3 (14) 0 All 45 patients who completed the placebo-controlled study (Study 1) continued treatment in an open-label, uncontrolled extension study (Study 2). All patients received ALDURAZYME 0.58 mg/kg of body weight once weekly for up to 182 weeks. The most serious adverse reactions reported with ALDURAZYME infusions in Study 2 were anaphylactic and hypersensitivity reactions [see Warnings and Precautions (5)]. The most common adverse reactions requiring intervention were infusion reactions reported in 49% (22 of 45) of patients treated with ALDURAZYME. The most commonly reported infusion reactions included rash (13%), flushing (11%), pyrexia (11%), headache (9%), abdominal pain or discomfort (9%), and injection site reaction (9%). Less commonly reported infusion reactions included nausea (7%), diarrhea (7%), feeling hot or cold (7%), vomiting (4%), pruritus (4%), arthralgia (4%), and urticaria (4%). Additional common adverse reactions included back pain and musculoskeletal pain.
Clinical Trials in Patients 6 Years and Younger
Study 3 was a 52-week, open-label, uncontrolled study of 20 MPS I patients, ages 6 months to 5 years old (at enrollment). Sixteen patients were clinically assessed as having the Hurler form, and 4 had the Hurler-Scheie form. All 20 patients received ALDURAZYME at 0.58 mg/kg of body weight once weekly for 26 weeks and up to 52 weeks. All patients were treated with antipyretics and antihistamines prior to the infusions.
The most commonly reported serious adverse events (regardless of relationship) reported with ALDURAZYME infusions in Study 3 were otitis media (20%), and central venous catheterization required for ALDURAZYME infusion (15%).
The nature and severity of infusion reactions were similar between the older and less severely affected patients in Studies 1 and 2, and the younger, more severely affected patients in Study 3. The most commonly reported adverse reactions in Study 3 were infusion reactions reported in 35% (7 of 20) of patients and included pyrexia (30%), chills (20%), blood pressure increased (10%), tachycardia (10%), and oxygen saturation decreased (10%). Other commonly reported infusion reactions occurring in ≥5% of patients were pallor, tremor, respiratory distress, wheezing, crepitations (pulmonary), pruritus, and rash.
6.2 Immunogenicity
As with all the therapeutic proteins, there is potential for immunogenicity. The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other laronidase products may be misleading.
In clinical trials, 99 of 102 patients (97%) treated with ALDURAZYME were positive for IgG antibodies to ALDURAZYME. No correlation was demonstrated between the presence of IgG anti-ALDURAZYME antibodies and therapeutic response (6 MWT and FVC) or the occurrence of hypersensitivity reactions. Potential for antibody neutralization of cellular uptake has not been assessed. No consistent association was demonstrated between the presence of antibodies that neutralize enzymatic activity and therapeutic response.
The data reflect the percentage of patients whose test results were considered positive for antibodies to ALDURAZYME using a specific enzyme-linked immunosorbent assay (ELISA) and confirmed by radio-immunoprecipitation (RIP). ALDURAZYME IgG antibodies were reported as titers. Drug specific antibody was detected in 42 of the 45 patients (93.3%) treated in Study 1 and Study 2. The mean time to seroconversion was 51 days in patients 6 years and older. In Study 3, all patients (100%) 5 years old or younger developed IgG antibodies against ALDURAZYME with a mean time to seroconversion of 26 days [see Clinical Studies (14)].
Nine patients in Study 1 and Study 2, collectively, who experienced severe infusion reactions were tested for ALDURAZYME-specific IgE antibodies and complement activation. IgE testing was performed by ELISA, and complement activation was measured by the Quidel Enzyme Immunoassay. One of the nine patients had an anaphylactic reaction consisting of urticaria and airway obstruction and tested positive for both ALDURAZYME-specific IgE binding antibodies and complement activation. None of the patients in the open-label clinical study of patients 5 years old or younger (Study 3) tested positive for IgE.
Other hypersensitivity reactions were also seen in patients receiving ALDURAZYME[see Adverse Reactions (6.1, 6.3)].
In the postmarketing setting, approximately 1% of patients experienced severe or serious infusion hypersensitivity reactions and tested positive for IgE. Of these IgE-positive patients, some have discontinued treatment, but some have been successfully re-challenged. The clinical significance of IgE antibodies has not been established.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ALDURAZYME. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with ALDURAZYME, severe and serious infusion reactions have been reported, some of which were life-threatening, including anaphylactic shock [see Boxed Warning and Warnings and Precautions (5)] and laryngeal edema.
Adverse reactions resulting in death reported in the postmarketing setting with ALDURAZYME treatment included cardiorespiratory arrest, respiratory failure, cardiac failure, and pneumonia. These events have been reported in MPS I patients with significant underlying disease.
Additional adverse reactions included fatigue, edema peripheral, erythema and cyanosis.
There have been a small number of reports of extravasation in patients treated with ALDURAZYME. There have been no reports of tissue necrosis associated with extravasation.
-
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
An MPS I Registry has been established and pregnant women with MPS I should be encouraged to enroll in the pregnancy sub-registry. For more information, visit www.registrynxt.com or call 1-800-745-4447 ext. 15500.
Risk Summary
Available data from published case reports and postmarketing experience with ALDURAZYME use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. No evidence of fetal harm has been observed in rats when laronidase was administered during organogenesis at doses up to 6.2 times the recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Pregnancy can exacerbate preexisting clinical manifestations of MPS and lead to adverse pregnancy outcomes for both mother and fetus.
Data
Animal Data
When laronidase was administered to pregnant female rats during organogenesis (gestation days [GD] 7-17) at doses of 0, 0.036, 0.36 or 3.6 mg/kg/day intravenously (equivalent to 7.3, 73.1, 730.8 units/kg/day) decreased maternal body weight gains and food consumption were observed with no corresponding effects on reproductive and litter parameters including number and distribution of corpora lutea, implantations and early and late resorptions at doses up to 3.6 mg/kg/day (6.2 times the recommended human dose of 0.58 mg/kg on a mg/kg basis). Laronidase has not been evaluated for effects on embryo-fetal development in any other species.
8.2 Lactation
Risk Summary
There are no available data on the presence of laronidase in human milk or the effects on milk production. No adverse effects have been reported in breastfed infants in a few postmarketing cases of laronidase use in lactating women. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ALDURAZYME and any potential adverse effects on the breastfed child from ALDURAZYME or from the underlying maternal condition.
Lactating women with MPS I are encouraged to enroll in the MPS I Registry. For more information, visit www.registrynxt.com or call 1-800-745-4447 ext. 15500.
8.4 Pediatric Use
The safety and effectiveness of ALDURAZYME was assessed in a 52-week, open-label, uncontrolled clinical study in 20 patients with MPS I, ages 6 months to 5 years old, and was found to be similar to the safety and effectiveness of ALDURAZYME in pediatric patients 6 to 18 years, and adults [see Adverse Reactions (6.1),Clinical Studies (14)].
-
10 OVERDOSAGE
There have been no reports of overdose with ALDURAZYME. In clinical studies, a small number of patients received doses up to 1.2 mg/kg body weight once weekly or 1.8 mg/kg body weight every other week. Adverse events reported in patients receiving 1.2 mg/kg body weight once weekly or 1.8 mg/kg body weight every other week were similar to the adverse events reported by patients treated with 0.58 mg/kg body weight once weekly.
-
11 DESCRIPTION
ALDURAZYME (laronidase) is a polymorphic variant of the human enzyme α‑L‑iduronidase that is produced by recombinant DNA technology in a Chinese hamster ovary cell line. α-L-iduronidase (glycosaminoglycan α-L-iduronohydrolase, EC 3.2.1.76) is a lysosomal hydrolase that catalyzes the hydrolysis of terminal α-L-iduronic acid residues of dermatan sulfate and heparan sulfate.
Laronidase is a glycoprotein with a molecular weight of approximately 83 kD. The predicted amino acid sequence of the recombinant form, as well as the nucleotide sequence that encodes it, are identical to a polymorphic form of human α-L-iduronidase. The recombinant protein is comprised of 628 amino acids after cleavage of the N-terminus and contains 6 N-linked oligosaccharide modification sites. Two oligosaccharide chains terminate in mannose-6-phosphate sugars. ALDURAZYME has a specific activity of approximately 172 U/mg.
ALDURAZYME, for intravenous infusion, is supplied as a sterile, nonpyrogenic, colorless to pale yellow, clear to slightly opalescent solution that must be diluted prior to administration in 0.9% Sodium Chloride Injection, USP. The solution in each vial contains a nominal laronidase concentration of 0.58 mg/mL and a pH of approximately 5.5. The extractable volume of 5 mL from each vial provides 2.9 mg laronidase, 43.9 mg sodium chloride, 63.5 mg sodium phosphate monobasic monohydrate, 10.7 mg sodium phosphate dibasic heptahydrate, and 0.05 mg polysorbate 80. ALDURAZYME does not contain preservatives; vials are for single dose only.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mucopolysaccharide storage disorders are caused by the deficiency of specific lysosomal enzymes required for the catabolism of glycosaminoglycans (GAG). Mucopolysaccharidosis I (MPS I) is characterized by the deficiency of α-L-iduronidase, a lysosomal hydrolase which catalyzes the hydrolysis of terminal α-L-iduronic acid residues of dermatan sulfate and heparan sulfate. Reduced or absent α-L-iduronidase activity results in the accumulation of the GAG substrates, dermatan sulfate and heparan sulfate, throughout the body and leads to widespread cellular, tissue, and organ dysfunction.
The rationale of ALDURAZYME therapy in MPS I is to provide exogenous enzyme for uptake into lysosomes and increase the catabolism of GAG. ALDURAZYME uptake by cells into lysosomes is most likely mediated by the mannose-6-phosphate-terminated oligosaccharide chains of laronidase binding to specific mannose-6-phosphate receptors.
Because many proteins in the blood are restricted from entry into the central nervous system (CNS) by the blood brain barrier, effects of intravenously administered ALDURAZYME on cells within the CNS cannot be inferred from activity in sites outside the CNS. The ability of ALDURAZYME to cross the blood brain barrier has not been evaluated in animal models or in clinical studies.
12.2 Pharmacodynamics
The pharmacodynamic effect of ALDURAZYME was assessed by reductions in urinary GAG levels. The responsiveness of urinary GAG to dosage alterations of ALDURAZYME is unknown, and the relationship of urinary GAG to other measures of clinical response has also not been established [see Clinical Studies (14)].
12.3 Pharmacokinetics
The pharmacokinetics of laronidase were evaluated in 6-year-old or older patients (N=10 to 12) with MPS I who received 0.58 mg/kg of body weight once weekly of ALDURAZYME as a 4-hour infusion in the placebo-controlled clinical study (Study 1). After the 1st, 12th, and 26th weekly infusions, the mean maximum plasma concentrations (Cmax) ranged from 1.2 to 1.7 mcg/mL for the 3 time points. The mean area under the plasma concentration-time curve (AUC∞) ranged from 4.5 to 6.9 μg hour/mL. The mean volume of distribution (Vz) ranged from 0.24 to 0.60 L/kg. Mean plasma clearance (CL) ranged from 1.7 to 2.7 mL/min/kg, and the mean elimination half-life (t1/2) ranged from 1.5 to 3.6 hours.
Most patients who received once weekly infusions of ALDURAZYME in Study 1 developed antibodies to laronidase by Week 12. Between Weeks 1 and 12, increases in the plasma clearance of laronidase were observed in some patients and appeared to be proportional to the antibody titer. At Week 26, plasma clearance of laronidase was comparable to that at Week 1, in spite of the continued and, in some cases, increased titers of antibodies.
The pharmacokinetics of laronidase were evaluated in 6-year-old or younger patients (N=7 to 9) with MPS I disease who received 0.58 mg/kg of body weight once weekly of ALDURAZYME as a 4-hour infusion in the open label clinical study (Study 3). After the 26th infusion, the 95% confidence interval of the geometric mean values of PK parameters ranged from 0.6 to 1.6 mcg/mL for the maximum plasma concentrations (Cmax), from 1.3 to 4.4 µg hour/mL for area under the plasma concentration-time curve (AUC∞), from 0.12 to 0.56 L/kg for volume of distribution (Vz), from 2.2 to 7.7 mL/min/kg for plasma clearance (CL), and from 0.3 to 1.9 hours for elimination half-life (t1/2).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the mutagenic and carcinogenic potential of laronidase have not been conducted.
Laronidase at intravenous doses up to 3.6 mg/kg (6.2 times the recommended human dose) was found to have no effect on the fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
14.1 Clinical Studies in Patients 6 Years and Older
Study 1 was a randomized, double-blind, placebo-controlled study in 45 patients with MPS I, ages 6 to 43 years old, including 1 patient with the Hurler form, 37 patients with Hurler-Scheie form, and 7 patients with Scheie form of MPS I. All patients had a baseline percent predicted forced vital capacity (FVC) less than or equal to 77%. Patients received ALDURAZYME at 0.58 mg/kg of body weight once weekly or placebo once weekly for 26 weeks. All patients were treated with antipyretics and antihistamines prior to each infusion.
The primary efficacy outcome assessments were percent predicted FVC and distance walked in 6 minutes (6-minute walk test). After 26 weeks, patients treated with ALDURAZYME showed improvement in percent predicted FVC and in 6-minute walk test compared to placebo-treated patients (see Table 4).
Table 4: Primary Efficacy Outcomes in the Placebo-controlled Study (Study 1) ALDURAZYME®
(N=22)Placebo
(N=23)Forced Vital Capacity (percent of predicted normal) Pretreatment Baseline Mean ± s.d. 48 ± 15 54 ± 16 Week 26 Mean ± s.d. 50 ± 17 51 ± 13 Change from Baseline to Week 26 Mean ± s.d. 1 ± 7 -3 ± 7 Median 1 -1 Difference in Change from
Baseline to Week 26 Between
GroupsMean 4 Median (95% CI) 2 (0.4, 7), p=0.02* 6-Minute Walk Distance (meters) Pretreatment Baseline Mean ± s.d. 319 ± 131 367 ± 114 Week 26 Mean ± s.d. 339 ± 127 348 ± 129 Change from Baseline to Week 26 Mean ± s.d. 20 ± 69 -18 ± 67 Median 28 -11 Difference in Change from
Baseline to Week 26 Between
GroupsMean 38 Median (95% CI) 39 (-2, 79), p=0.07* Evaluations of bioactivity were changes in liver size and urinary GAG levels. Liver size and urinary GAG levels decreased in patients treated with ALDURAZYME compared to patients treated with placebo. No patient in the group receiving ALDURAZYME reached the normal range for urinary GAG levels during this 6-month study.
Study 2 was a 182-week, open-label, uncontrolled extension study of all 45 patients who completed Study 1. Patients received ALDURAZYME at 0.58 mg/kg body weight once weekly. For patients treated with ALDURAZYME, the mean increase in 6-minute walk test distance was maintained for an additional 182 weeks through completion of Study 2.
At the end of Study 2, the decrease in mean urinary GAG was similar to the decrease in urinary GAG reported in ALDURAZYME-treated patients at the end of Study 1. The relationship of urinary GAG to other measures of clinical response has not been established.
14.2 Clinical Studies in Patients 6 Years and Younger
Study 3 was a 52-week, open-label, uncontrolled clinical study in 20 patients with MPS I, ages 6 months to 5 years old (at enrollment), including 16 patients (80%) with the Hurler form and 4 patients (20%) with the Hurler-Scheie form. All 20 patients received ALDURAZYME at 0.58 mg/kg of body weight once weekly for 26 weeks. After 26 weeks of treatment, 16 patients continued to receive 0.58 mg/kg of body weight once weekly through Week 52, and 4 patients received 1.16 mg/kg of body weight once weekly from Week 26 through Week 52.
Reduction in mean urinary GAG was demonstrated at Week 13 and was maintained through Week 52. No patient receiving ALDURAZYME reached the normal range for urinary GAG levels during this 52-week study. Changes in urinary GAG levels in children 6 years and younger were similar to changes reported in older patients in Studies 1 and 2 (6 through 43 years old). The relationship of urinary GAG to other measures of clinical response has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ALDURAZYME is supplied as a sterile colorless to pale yellow, clear to slightly opalescent solution in single-dose, clear Type I glass 5 mL vials, containing 2.9 mg/5 mL mg laronidase. The closure consists of a siliconized butyl stopper and an aluminum seal with a plastic flip-off cap.
NDC: 58468-0070-1, 5 mL vial
Refrigerate vials of ALDURAZYME at 2°C to 8°C (36°F to 46°F). Do not freeze or shake. Protect from light. This product contains no preservatives.
-
17 PATIENT COUNSELING INFORMATION
Anaphylaxis, Hypersensitivity and Infusion Reactions
Inform the patient or caregiver that hypersensitivity reactions, including life-threatening anaphylaxis, and infusion reactions may occur with ALDURAZYME treatment. Advise the patient or caregiver to report immediately to a healthcare provider if signs or symptoms of a hypersensitivity or infusion reaction occur during infusion of ALDURAZYME. Hypersensitivity reactions may also occur up to 3 hours following an infusion of ALDURAZYME [see Warnings and Precautions (5.1,5.4)].
Cardiac and Respiratory Adverse Reactions
Advise the patient or caregiver to report immediately to a healthcare provider if signs or symptoms of cardiac or respiratory decompensation occur during or following an infusion [see Warnings and Precautions (5.2, 5.3)] Inform patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep to have these treatments readily available during infusion or extreme drowsiness/sleep induced by antihistamine use.
Registry
Patients should be informed that a registry for MPS I patients has been established in order to better understand the MPS I disease, and to track clinical outcomes of patients with MPS I over time. The MPS I Registry also monitors the effect of Aldurazyme on pregnant women, lactating women, and their infants. Patients should be encouraged to participate and advised that their participation is voluntary and may involve long-term follow-up. Information regarding the registry program may be found at www.registrynxt.com or by calling 1‑800‑745‑4447 ext. 15500.
ALDURAZYME is manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
US License Number 1649
ALDURAZYME is distributed by:
Genzyme CorporationCambridge, MA 02142
1-800-745-4447 (phone)
ALDURAZYME® is a registered trademark of BioMarin/Genzyme LLC. All rights reserved. -
PACKAGE LABEL
NDC: 58468-0070-1
ALDURAZYME®
(LARONIDASE)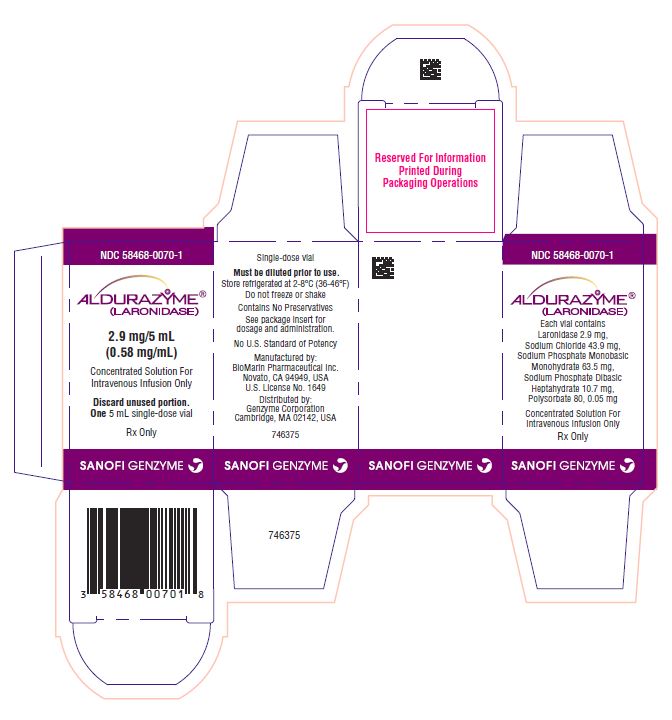
2.9 mg/5 mL
(0.58 mg/mL)Concentrated Solution For
Intravenous Infusion OnlyDiscard unused portion.
One 5 mL single-dose vial
Rx Only
genzyme
Single-dose vial
Must be diluted prior to use.
Store refrigerated at 2-8°C (36-46°F)
Do not freeze or shakeContains No Preservatives
See package insert for
dosage and administration.No U.S. Standard of Potency
Manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949, USA
U.S. License No. 1649Distributed by:
Genzyme Corporation
Cambridge, MA 02142, USAgenzyme
NDC: 58468-0070-1
ALDURAZYME®
(LARONIDASE)Each vial contains
Laronidase 2.9 mg,
Sodium Chloride 43.9 mg,
Sodium Phosphate Monobasic Monohydrate 63.5 mg,
Sodium Phosphate Dibasic Heptahydrate 10.7 mg,
Polysorbate 80, 0.05 mg, in 5mLConcentrated Solution For
Intravenous Infusion OnlyRx Only
genzyme
LOT
EXP

Single-dose vial
See package insert for
dosage and administrationStore at 2-8°C (36-46°F)
Manufactured by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
U.S. License No. 1649Distributed by:
Genzyme Corporation
Cambridge, MA 02142genzyme
NDC: 58468-0070-1
ALDURAZYME®
(LARONIDASE)2.9 mg/5 mL (0.58 mg/mL)
Concentrated Solution For
Intravenous Infusion OnlyMust be diluted prior to use.
Rx Only
LOT:
EXP: -
INGREDIENTS AND APPEARANCE
ALDURAZYME
laronidase injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 58468-0070 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LARONIDASE (UNII: WP58SVM6R4) (LARONIDASE - UNII:WP58SVM6R4) LARONIDASE 2.9 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58468-0070-1 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 04/30/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125058 04/30/2003 Labeler - Genzyme Corporation (025322157)
Trademark Results [ALDURAZYME]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALDURAZYME 77118358 3380455 Live/Registered |
BIOMARIN/GENZYME LLC 2007-02-28 |
 ALDURAZYME 75927731 2640306 Live/Registered |
BioMarin/Genzyme LLC 2000-02-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.