Hempvana Endtag by Telebrands Corp / King Bio Inc
Hempvana Endtag by
Drug Labeling and Warnings
Hempvana Endtag by is a Homeopathic medication manufactured, distributed, or labeled by Telebrands Corp, King Bio Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
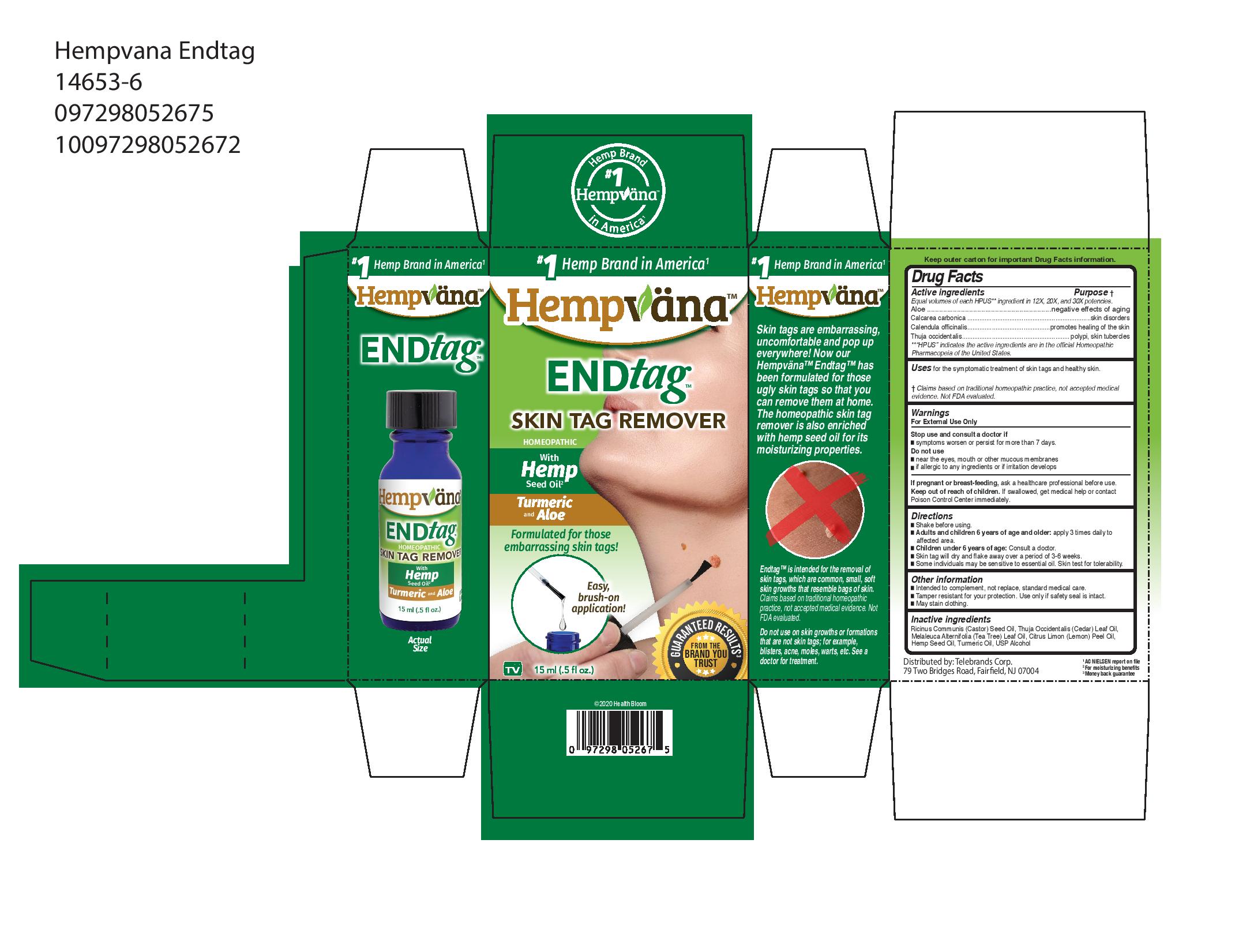
HEMPVANA ENDTAG- hempvana endtag liquid
Telebrands Corp
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Equal Volumes of each HPUS ** ingredient in 12x, 20x, and 30x potencies.
Aloe....
Calcarea carbonica
Calendula officinalis
Thuja occidentalis
**HPUS indicates the active ingredie ts are in the official Homeopathic Pharmacopeia of the United States
Purpose
Aloe....negative effects of againg
Calcarea carbonica.....skin disorders
Calendula officinalis.....promotes healing of the skin
Thuja occidentalis.....polypi, skin, tubercles
Uses
for the symptomatic treatment of skin tags and healthy skin.
+claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Do not use
- near the eyes, mouth or other mucous membranes
- if allergeic to any ingrediens or if irritation develops
Keep out of reach of children. If swallowed, get medical help or contact a poison control center immediately.
Directions
- Shake before using
- Adults and children 6 years of age and older: apply 3 times daily to affected area.
- Children under 6 years of age: Consult a doctor.
- Skin tag will dry and flake away over a period of 3-6 weeks.
- Some individuals may be senstitive to essential oil. Skin test for tolerability.
Other Information
- Intended to compliment, not replace, standard medcial care
- Tamper resistant for your protection. Use only if safety seal is intact.
- May stain clothing.
| HEMPVANA ENDTAG
hempvana endtag liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Telebrands Corp (177266558) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| King Bio Inc | 617901350 | manufacture(73287-010) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.