DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
DIANEAL LOW CALCIUM WITH DEXTROSE by
Drug Labeling and Warnings
DIANEAL LOW CALCIUM WITH DEXTROSE by is a Prescription medication manufactured, distributed, or labeled by Vantive US Healthcare LLC, Baxter Healthcare (Guangzhou) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
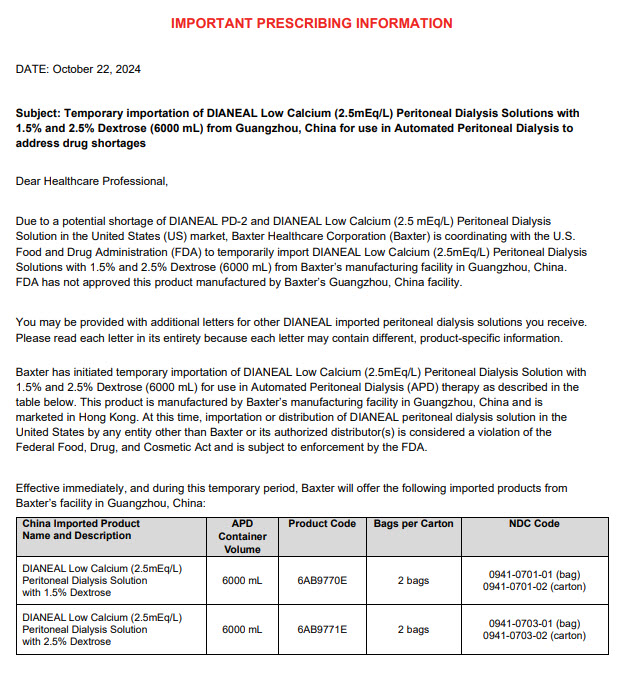
- Health Care Provider Letter
-
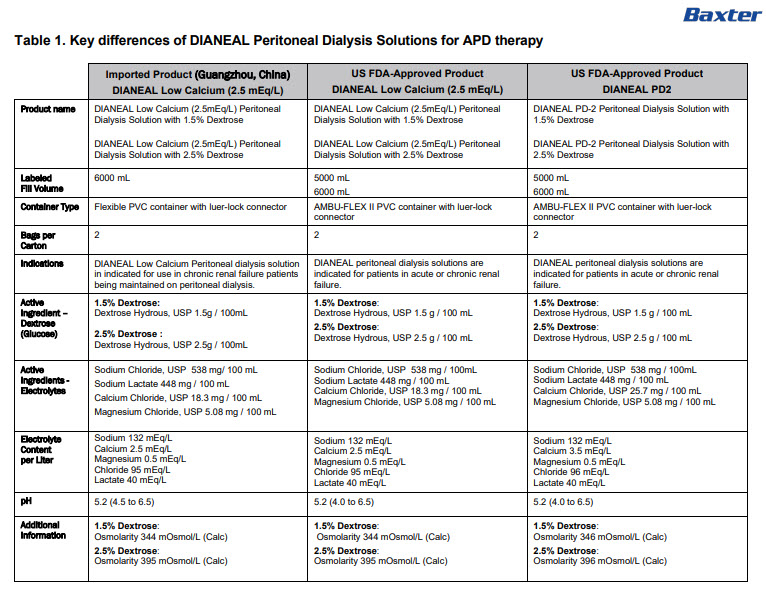
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
6AB9770E 6000ml
(APPROX 225ml EXCESS)BaxterLogo
Dianeal ®Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 1.5% DextroseEACH 100ml CONTAINS1.5 g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 TO 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40
OSMOLARITY344 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE
DIRECTION OF A PHYSICIANREAD PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
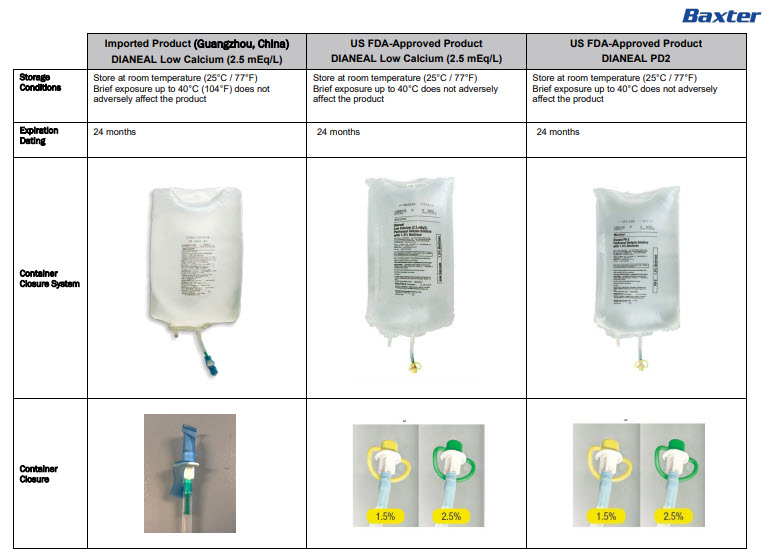
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER 25°C) UNTIL READY TO USE
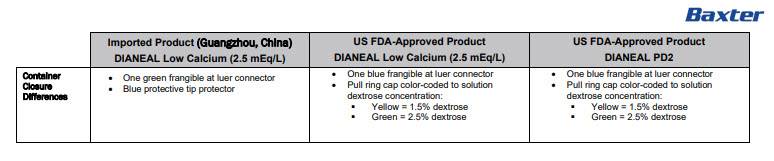
AVOID EXCESSIVE HEAT SEE INSERTAmbu-Flex™ IIICONTAINER PL-146 ®PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFI LIATE OF BAXTER WORLD TRADE INC USA)
HK-42530
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD,Guangzhou, P.R. ChinaLow Calcium 1.5% Dextrose
1.5 LOW CALCIUM WITH 1.5% DEXTROSE 6000mlX2
LOT G00000000 EXP JAN 00 6AB9770E S/N 00006AB9771E 6000ml
(APPROX 225ml EXCESS)BaxterLogo
Dianeal ®Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 2.5% DextroseEACH 100ml CONTAINS2.5 g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 TO 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40
OSMOLARITY395 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE
DIRECTION OF A PHYSICIANREAD PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER 25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTAmbu-Flex™ IIICONTAINER PL-146 ®PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFI LIATE OF BAXTER WORLD TRADE INC USA)
HK-42531
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD,Guangzhou, P.R. ChinaLow Calcium 1.5% Dextrose
2.5 LOW CALCIUM WITH 2.5% DEXTROSE 6000mlX2
LOT G00000000 EXP JAN 00 6AB9771E S/N 0000 -
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0701 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0701-02 2 in 1 CARTON 11/07/2024 12/31/2026 1 NDC: 0941-0701-01 6000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/07/2024 12/31/2026 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0703 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0703-02 2 in 1 CARTON 11/07/2024 12/31/2026 1 NDC: 0941-0703-01 6000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/07/2024 12/31/2026 Labeler - Vantive US Healthcare LLC (119181963) Establishment Name Address ID/FEI Business Operations Baxter Healthcare (Guangzhou) Co., Ltd 421040114 manufacture(0941-0701, 0941-0703) , analysis(0941-0701, 0941-0703) , label(0941-0701, 0941-0703) , pack(0941-0701, 0941-0703) , sterilize(0941-0701, 0941-0703)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.