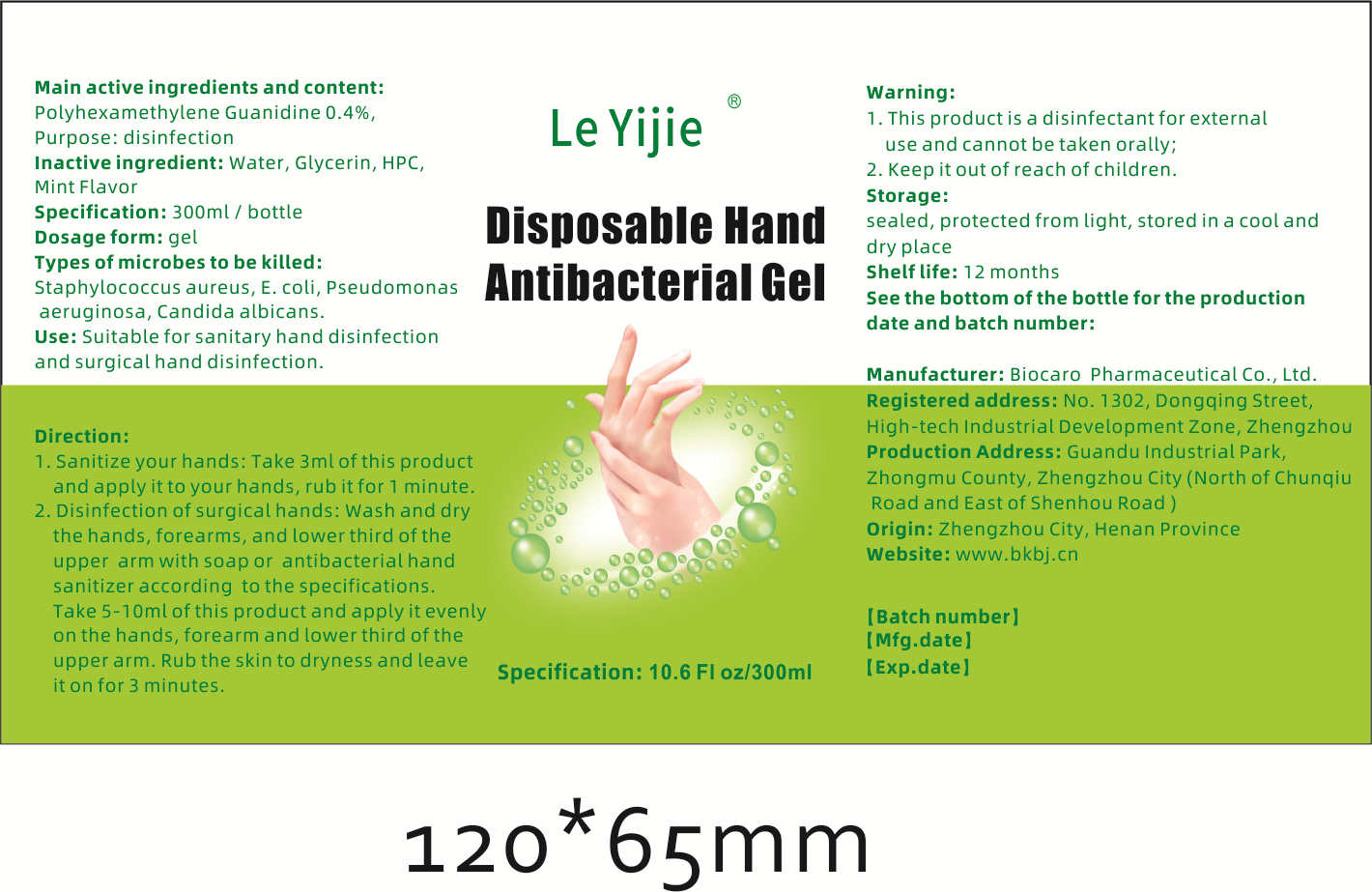
Disposable Hand Antibacterial Gel
Disposable Hand Antibacterial Gel by
Drug Labeling and Warnings
Disposable Hand Antibacterial Gel by is a Otc medication manufactured, distributed, or labeled by Biocaro Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DISPOSABLE HAND ANTIBACTERIAL GEL- disposable hand antibacterial gel gel
Biocaro Pharmaceutical Co., Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Disposable Hand Antibacterial Gel
Warnings
Warning:1. This product is a disinfectant for externaluse and cannot be taken orally;
2. Keep it out of reach of children
Do not use
- 1. This product is a disinfectant for external use and cannot be taken orally;2. Keep it out of reach of children
Directions
- Direction:1. Sanitize your hands: Take 3ml of this productand apply it to your hands, rub it for 1 minute.2. Disinfection of surgical hands: Wash and drythe hands, forearms, and lower third of theupper arm with soap or antibacterial handsanitizer according to the specificationsTake 5-10ml of this product and apply it evenon the hands, forearm and lower third of theupper arm. Rub the skin to dryness and leaveit on for 3 minutes
| DISPOSABLE HAND ANTIBACTERIAL GEL
disposable hand antibacterial gel gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Biocaro Pharmaceutical Co., Ltd (527295318) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biocaro Pharmaceutical Co., Ltd | 527295318 | manufacture(78872-005) | |
Revised: 4/2021
Document Id: c0d920b0-e9ec-5ee4-e053-2995a90af65f
Set id: a829f10f-b28b-0e62-e053-2995a90a0646
Version: 4
Effective Time: 20210426