Medi-First Alcohol Wipes by Unifirst First Aid Corporation Medi-First Alcohol Wipes
Medi-First Alcohol Wipes by
Drug Labeling and Warnings
Medi-First Alcohol Wipes by is a Otc medication manufactured, distributed, or labeled by Unifirst First Aid Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
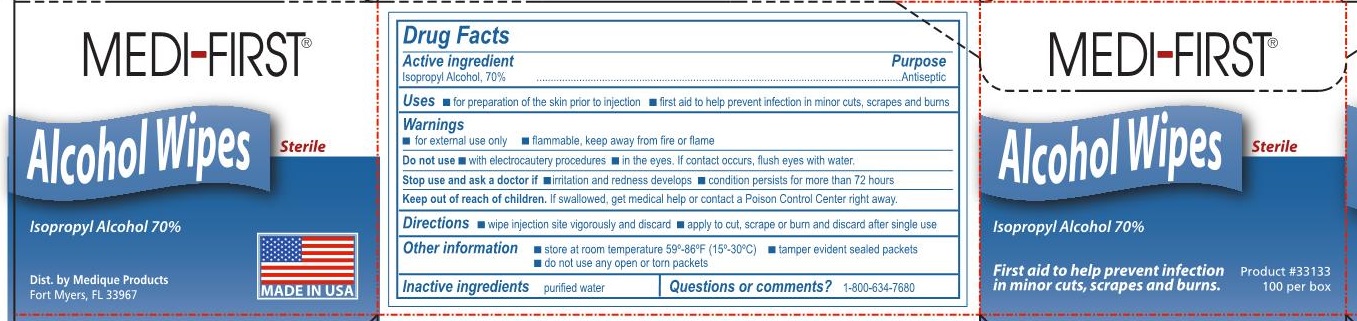
MEDI-FIRST ALCOHOL WIPES- isopropyl alcohol, 70% swab
Unifirst First Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Medi-First Alcohol Wipes
Uses
- for preparation of the skin prior to injection
- first aid to help prevent infection in minor cuts, scrapes and burns
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wipe injection site vigorously and discard
- apply to cut, scrape or burn and discard after single use
| MEDI-FIRST ALCOHOL WIPES
isopropyl alcohol, 70% swab |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Unifirst First Aid Corporation (832947092) |
Revised: 3/2023
Document Id: f5d6ee54-1fa1-c87b-e053-2a95a90a14ec
Set id: a83647fa-fadd-a3fa-e053-2995a90a6450
Version: 4
Effective Time: 20230301