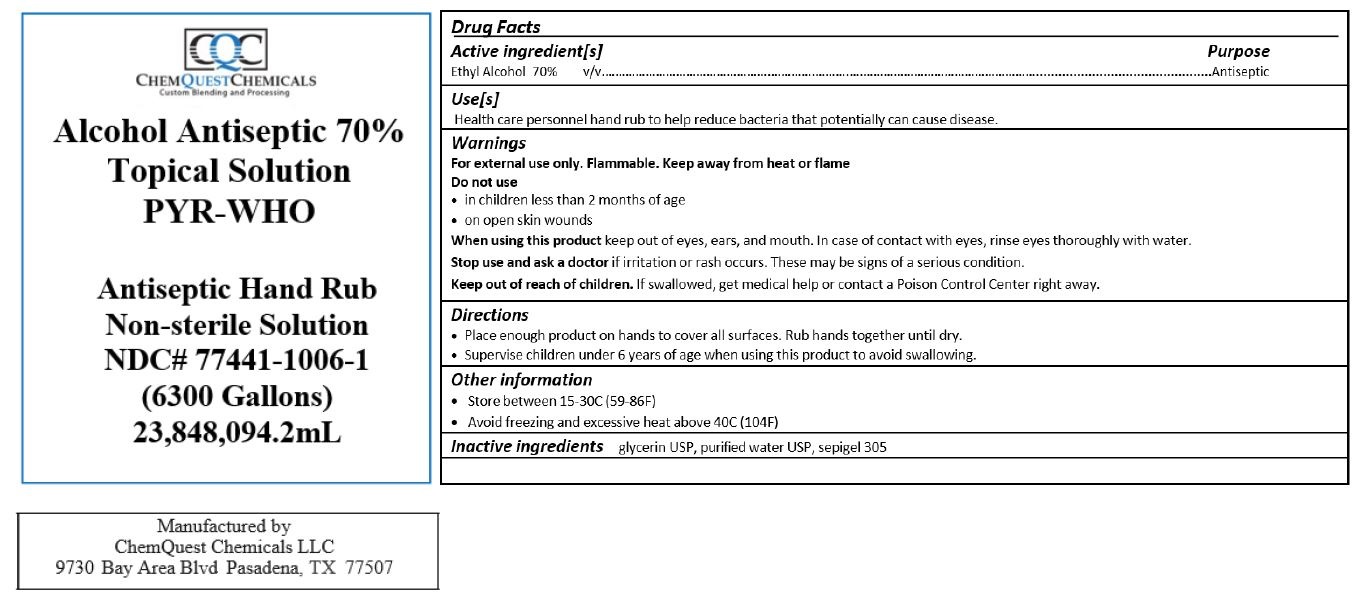
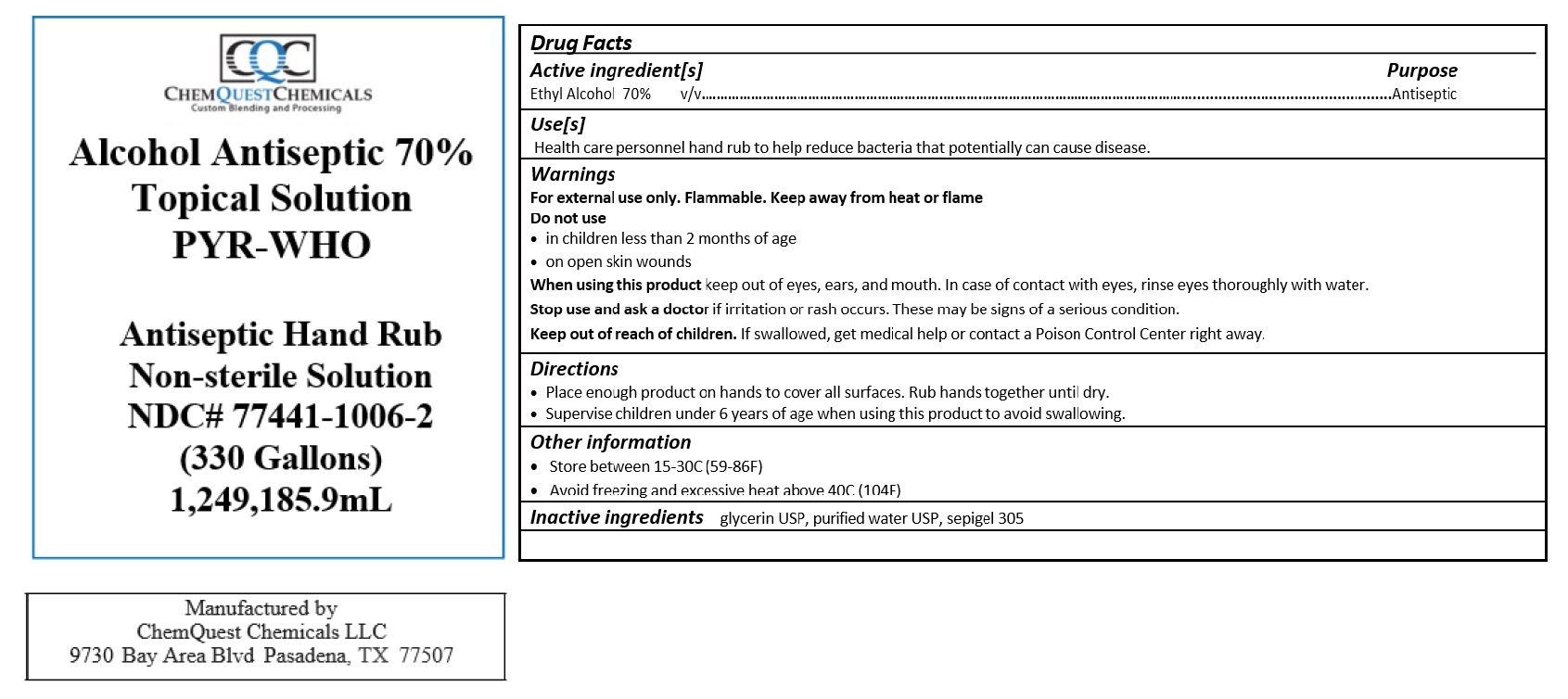
Alcohol Antiseptic 70% Topical Solution PYR-WHO
Antiseptic Hand Rub by
Drug Labeling and Warnings
Antiseptic Hand Rub by is a Otc medication manufactured, distributed, or labeled by ChemQuest Chemicals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC HAND RUB- alcohol liquid
ChemQuest Chemicals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Alcohol Antiseptic 70% Topical Solution PYR-WHO
Warnings
For external use only. Flammable. Keep away from heat or flame
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
| ANTISEPTIC HAND RUB
alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ChemQuest Chemicals LLC (835052051) |
| Registrant - ChemQuest Chemicals LLC (835052051) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ChemQuest Chemicals LLC | 835052051 | manufacture(77441-1006) , pack(77441-1006) , label(77441-1006) | |
Revised: 8/2022
Document Id: e68c4fdf-c40a-304e-e053-2a95a90a61cf
Set id: a8409f49-a26c-705a-e053-2995a90ab334
Version: 3
Effective Time: 20220818