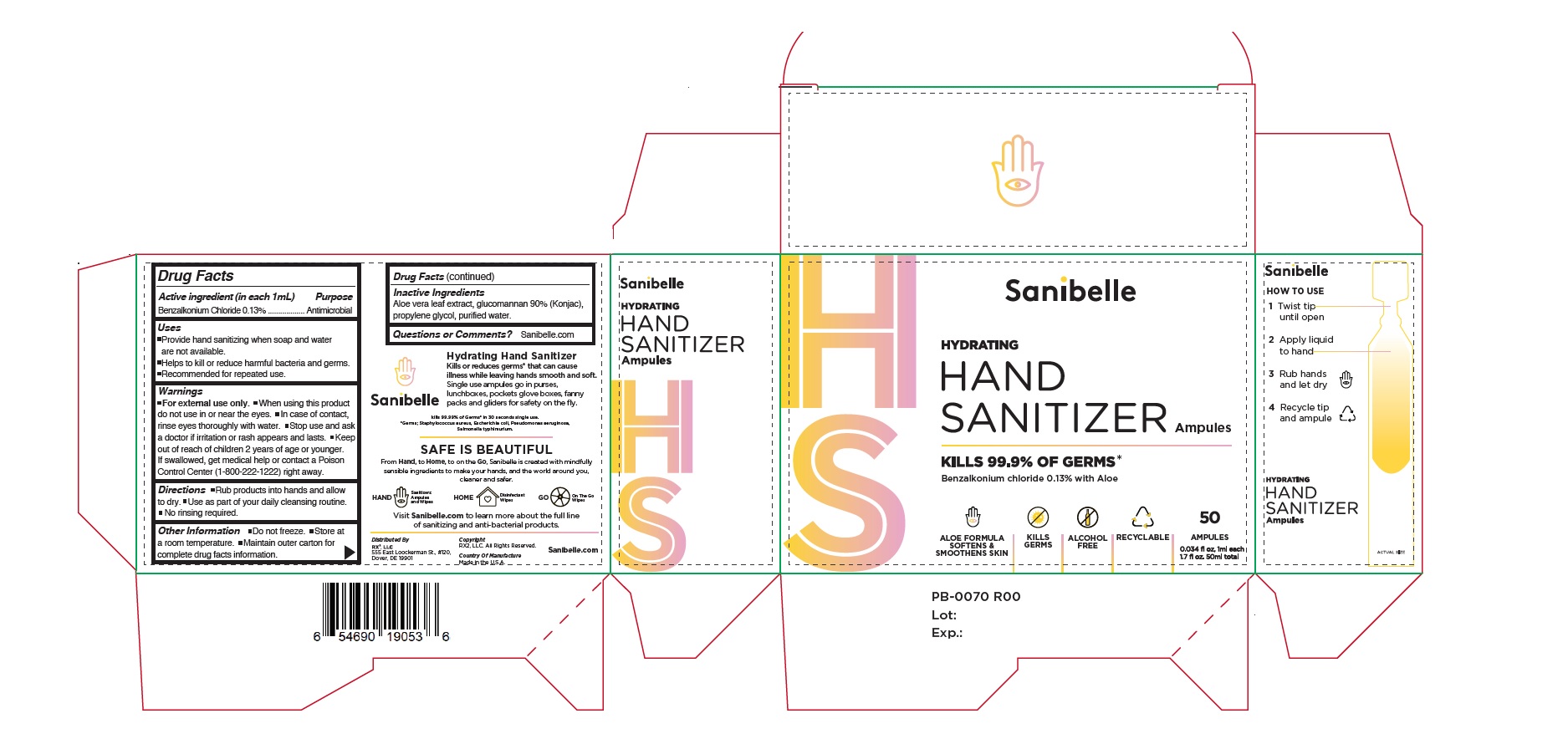
Sanibelle Hand Sanitizer 50 Single Use vials per 1mL ea
Sanibelle Hand Sanitizer 50 Single Use Vials x 1mL ea by
Drug Labeling and Warnings
Sanibelle Hand Sanitizer 50 Single Use Vials x 1mL ea by is a Otc medication manufactured, distributed, or labeled by Unipharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANIBELLE HAND SANITIZER 50 SINGLE USE VIALS X 1ML EA- benzalkonium chloride solution
Unipharma, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Sanibelle Hand Sanitizer 50 Single Use vials per 1mL ea
Use
- Provide Hand sanitizing when soap and water are not available.
- Helps to kill or reduce harmful bacteria and germs.
- Recommended for repeated use.
Warnings
For external use only
Directions
- Rub product into hands and allow to dry
- Use as part of your daily cleansing routine
- No rinsing required
| SANIBELLE HAND SANITIZER 50 SINGLE USE VIALS X 1ML EA
benzalkonium chloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Unipharma, LLC (079524332) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Unipharma, LLC | 079524332 | manufacture(70302-073) | |
Revised: 6/2020
Document Id: a87510bd-d82c-5e20-e053-2995a90a17bd
Set id: a8760d26-3892-ddd9-e053-2a95a90a2924
Version: 1
Effective Time: 20200619
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.