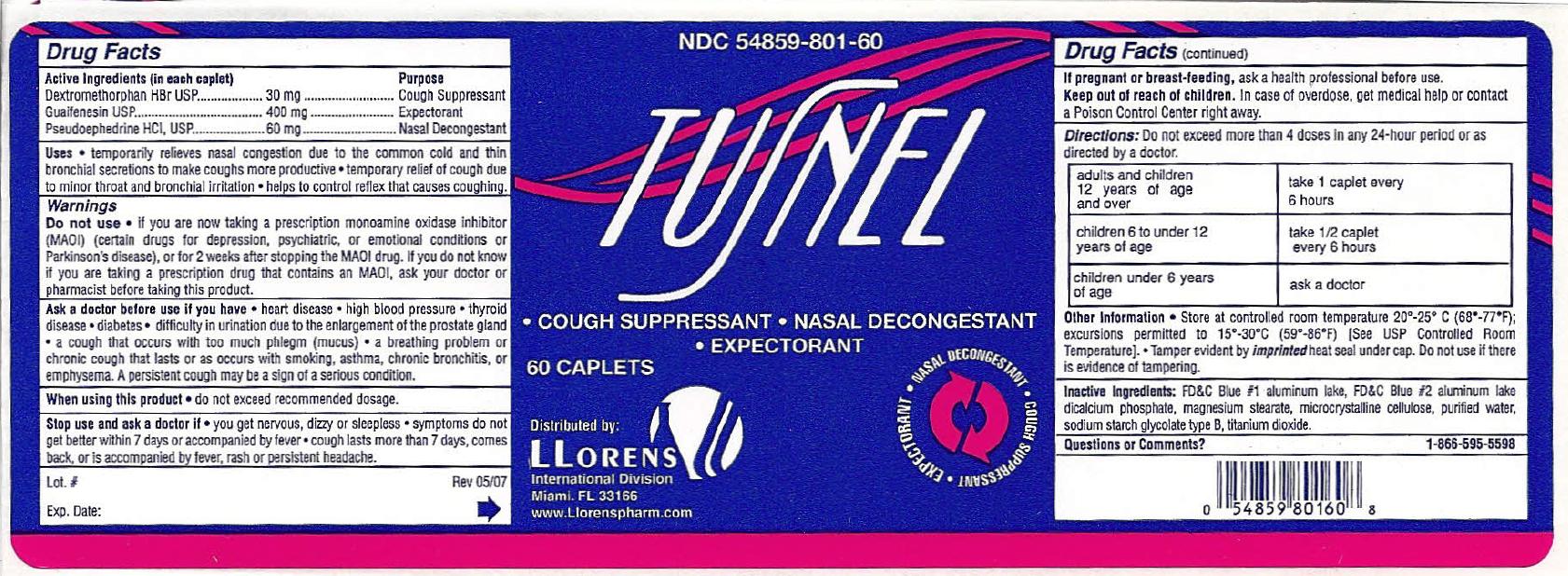
TUSNEL- dextromethorphan hbr, guaifenesin, pseudoephedrine hcl tablet
Tusnel by
Drug Labeling and Warnings
Tusnel by is a Otc medication manufactured, distributed, or labeled by LLORENS PHARMACEUTICALS INTERNATIONAL DIVISION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active Ingredients (In each tablet) Purpose
Dextromethorphan HBr ................... 30 mg ................... Cough Suppressant
Guaifenesin .................................. 400 mg .................. Expectorant
Pseudoephedrine HCl .................... 60 mg .................... Nasal Decongestant
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to the enlargement of the prostate gland
- a cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema. A persistent cough may be a sign of a serious condition.
When using this product do not exceed recommended dosage.
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days or accompanied by fever
- cough lasts more than 7 days, comes back or is accompanied by fever, rash or persistent headache
-
DO NOT USE
Do not use
- if you are now taking a prescription monamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you are taking a prescription drug that contains on MAOI, ask your doctor or pharmacist before taking this product.
- if you are now taking a prescription monamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you are taking a prescription drug that contains on MAOI, ask your doctor or pharmacist before taking this product.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TUSNEL
dextromethorphan hbr, guaifenesin, pseudoephedrine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54859-801 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) SODIUM STARCH GLYCOLATE TYPE B POTATO (UNII: 27NA468985) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL Size 20mm Flavor Imprint Code TUSNEL Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54859-801-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/01/2007 Labeler - LLORENS PHARMACEUTICALS INTERNATIONAL DIVISION (037342305)
Trademark Results [Tusnel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TUSNEL 87532022 5527955 Live/Registered |
LLORENS PHARMACEUTICAL INTERNATIONAL DIVISION 2017-07-18 |
 TUSNEL 86162169 not registered Dead/Abandoned |
Llorens Pharmaceutical International Division, Inc. 2014-01-10 |
 TUSNEL 85690038 not registered Dead/Abandoned |
Pharmaceutical Generic Developers Inc. USA 2012-07-30 |
 TUSNEL 77666900 not registered Dead/Abandoned |
Llorens Pharmaceutical International Divison, Inc 2009-02-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.