INCRELEX- mecasermin injection, solution
Increlex by
Drug Labeling and Warnings
Increlex by is a Prescription medication manufactured, distributed, or labeled by Ipsen Biopharmaceuticals, Inc., Hospira, Inc., Tjoapack Netherlands B.V., Baxter Oncology GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INCRELEX® safely and effectively. See full prescribing information for INCRELEX®.
INCRELEX® (mecasermin) injection, for subcutaneous use
Initial U.S. Approval: 2005RECENT MAJOR CHANGES
INDICATIONS AND USAGE
INCRELEX (mecasermin) injection is indicated for the treatment of growth failure in pediatric patients 2 years of age and older with severe primary IGF-1 deficiency or with growth hormone (GH) gene deletion who have developed neutralizing antibodies to GH. (1.1) (1)
Limitations of use: INCRELEX is not a substitute to GH for approved GH indications. (1)
DOSAGE AND ADMINISTRATION
- INCRELEX should be administered subcutaneously. (2.2)
- Injection sites should be rotated to avoid lipohypertrophy. (2.2)
- Recommended starting dose: 0.04 to 0.08 mg/kg (40 to 80 micrograms/kg) twice daily. If well-tolerated for at least one week, the dose may be increased by 0.04 mg/kg per dose, to the maximum dose of 0.12 mg/kg given twice daily. (2.1)
DOSAGE FORMS AND STRENGTHS
- INCRELEX is a sterile solution supplied in a multiple dose glass vial at a concentration of 10 mg per mL (40 mg per vial). (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypoglycemia: INCRELEX should be administered shortly before or after a meal or snack, because it has insulin-like hypoglycemic effects. (5.1)
- Hypersensitivity and Allergic Reactions, including Anaphylaxis: A low number of cases indicative of anaphylaxis requiring hospitalization have been reported. Parents and patients should be informed that such reactions are possible and that if a systemic allergic reaction occurs, treatment should be interrupted and prompt medical attention should be sought. (5.2)
- Intracranial Hypertension: Funduscopic examination is recommended at the initiation and periodically during the course of INCRELEX therapy. (5.3)
- Lymphoid Tissue Hypertrophy (tonsillar/adenoidal hypertrophy): Patients should have periodic examinations to rule out potential complications and receive appropriate treatment if necessary. (5.4)
- Slipped Capital Femoral Epiphysis (SCFE): Evaluate any child with onset of a limp or hip/knee pain for possible SCFE. (5.5)
- Progression of Scoliosis: Monitor any child with scoliosis for progression of the spine curve. (5.6)
- Malignant Neoplasia: Several cases of malignant neoplasia have been observed in pediatric patients treated with INCRELEX. Therapy should be discontinued if evidence of malignant neoplasia develops and appropriate expert medical care sought. (5.7)
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution: Benzyl alcohol, a preservative in INCRELEX, has been associated with serious adverse reactions, including death, in neonates and infants. Use of INCRELEX in infants is not recommended. (5.8)
ADVERSE REACTIONS
Common INCRELEX-related adverse reactions in clinical trials include: hypoglycemia, local and systemic hypersensitivity, tonsillar hypertrophy (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Ipsen Biopharmaceuticals, Inc. at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
- Pediatric Use: Safety and effectiveness has not been established in children less than 2 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
5.2 Hypersensitivity and Allergic Reactions, including Anaphylaxis
5.3 Intracranial Hypertension
5.4 Lymphoid Tissue Hypertrophy
5.5 Slipped Capital Femoral Epiphysis
5.6 Progression of Preexisting Scoliosis
5.7 Malignant Neoplasia
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
6.3 Post-Marketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Effects of INCRELEX Treatment in Children with Severe Primary Insulin-like Growth Factor-1 Deficiency (Severe Primary IGFD)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Severe Primary IGF-1 Deficiency (Primary IGFD)
INCRELEX is indicated for the treatment of growth failure in pediatric patients 2 years of age and older with:
- severe primary IGF-1 deficiency or
- growth hormone (GH) gene deletion who have developed neutralizing antibodies to GH.
Severe Primary IGF-1 deficiency (IGFD) is defined by:
- height standard deviation score ≤ –3.0 and
- basal IGF-1 standard deviation score ≤ –3.0 and
- normal or elevated growth hormone (GH).
Limitations of use:
INCRELEX is not a substitute to GH for approved GH indications.
INCRELEX is not indicated for use in patients with secondary forms of IGF-1 deficiency, such as GH deficiency, malnutrition, hypothyroidism, or chronic treatment with pharmacologic doses of anti-inflammatory corticosteroids.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
- Treatment with INCRELEX should be supervised by a physician who is experienced in the diagnosis and management of pediatric patients with short stature associated with severe primary IGF-1 deficiency or with growth hormone gene deletion and who have developed neutralizing antibodies to growth hormone.
- The dosage of INCRELEX should be individualized for each patient. The recommended starting dose of INCRELEX is 0.04 to 0.08 mg/kg (40 to 80 micrograms/kg) twice daily by subcutaneous injection. If well-tolerated for at least one week, the dose may be increased by 0.04 mg/kg per dose, to the maximum dose of 0.12 mg/kg given twice daily [see Warnings and Precautions (5.1 and 5.7)].
- Preprandial glucose monitoring is recommended at treatment initiation and until a well-tolerated dose is established. If frequent symptoms of hypoglycemia or severe hypoglycemia occur, preprandial glucose monitoring should continue. If hypoglycemia occurs with recommended doses despite adequate food intake, the dose should be reduced. INCRELEX should be administered shortly before or after (± 20 minutes) a meal or snack. If the patient is unable to eat shortly before or after a dose for any reason, that dose of INCRELEX should be withheld.
- If one or more doses of INCRELEX is missed, do not increase the subsequent doses to make up for omitted doses.
2.2 Administration
INCRELEX is administered by subcutaneous injection only. Do not administer intravenously.
INCRELEX injection sites should be rotated to a different site (upper arm, thigh, buttock or abdomen) with each injection to help prevent lipohypertrophy.
INCRELEX should be administered using sterile disposable syringes and needles. The syringes should be of small enough volume so that the prescribed dose can be withdrawn from the vial with accuracy.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
If using syringes that measure dose in units, doses in mg/kg must be converted to units using the following formula: Weight (kg) × Dose (mg/kg) × 1 mL/10 mg × 100 units/1 mL = units/injection.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Known Hypersensitivity
INCRELEX should not be used by patients who are allergic to mecasermin (rhIGF-1) or any of the inactive ingredients in INCRELEX, or who have experienced a severe hypersensitivity to INCRELEX [see Warnings and Precautions (5.2) and Adverse Reactions (6.3)].
- Closed Epiphyses
INCRELEX should not be used for growth promotion in patients with closed epiphyses.
- Malignant Neoplasia
INCRELEX is contraindicated in pediatric patients with malignant neoplasia or a history of malignancy [see Warnings and Precautions (5.7) and Adverse Reactions (6.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypoglycemia
Because INCRELEX has insulin-like hypoglycemic effects it should be administered shortly before or after (± 20 minutes) a meal or snack. Glucose monitoring and INCRELEX dose titration are recommended until a well tolerated dose is established [see Dosage and Administration (2.1)] and subsequently as medically indicated. Special attention should be paid to small children because their oral intake may not be consistent. Patients should avoid engaging in any high-risk activities (e.g., driving, exercise, etc.) within 2 to 3 hours after dosing, particularly during the initiation of INCRELEX treatment until tolerability and a stable dose have been established [see Adverse Reactions (6.1)]. INCRELEX should not be administered when the meal or snack is omitted. The dose of INCRELEX should never be increased to make up for one or more omitted doses.
5.2 Hypersensitivity and Allergic Reactions, including Anaphylaxis
Allergic reactions to INCRELEX have been reported post-marketing. They range from localized (injection site) reactions to systemic reactions, including anaphylaxis requiring hospitalization. Parents and patients should be informed that such reactions are possible and that if a systemic allergic reaction occurs, treatment should be interrupted and prompt medical attention should be sought. [see Contraindications (4) and Adverse Reactions (6.3)]
5.3 Intracranial Hypertension
Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea and/or vomiting have occurred in patients treated with INCRELEX. IH-associated signs and symptoms resolved after interruption of dosing. Funduscopic examination is recommended at the initiation and periodically during the course of INCRELEX therapy. [see Adverse Reactions (6.3)].
5.4 Lymphoid Tissue Hypertrophy
Lymphoid tissue (e.g., tonsillar and adenoidal) hypertrophy associated with complications, such as snoring, sleep apnea, and chronic middle-ear effusions have been reported with the use of INCRELEX. Patients should have periodic examinations to rule out such potential complications and receive appropriate treatment if necessary [see Adverse Reactions (6.3)].
5.5 Slipped Capital Femoral Epiphysis
Slipped capital femoral epiphysis can occur in patients who experience rapid growth. Any pediatric patient with the onset of a limp or complaints of hip or knee pain during INCRELEX therapy should be carefully evaluated.
5.6 Progression of Preexisting Scoliosis
Progression of scoliosis may occur in patients who experience rapid growth. Because INCRELEX increases growth rate, patients with a history of scoliosis who are treated with INCRELEX should be monitored for progression of scoliosis.
5.7 Malignant Neoplasia
There have been postmarketing reports of malignant neoplasms in pediatric patients who have received treatment with INCRELEX [see Adverse Reactions (6.3)]. The cases of malignant neoplasms represented a variety of different malignancies. It is unknown whether there is any relationship between INCRELEX therapy and new occurrence of neoplasia. The occurrence of neoplasia was mostly reported in patients with rare genetic conditions of short stature associated with an increased risk of cancer, or in patients with other cancer predisposing conditions. The tumors were observed also more frequently in patients who received INCRELEX at higher than recommended doses, or at doses that produced serum IGF-1 levels above the normal reference ranges for age and sex. Monitor all patients receiving INCRELEX carefully for development of neoplasms. Advise patients/caregivers to report development of new neoplasms. If malignant neoplasia develops, discontinue INCRELEX treatment [see Contraindications (4)].
5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preserved Solution
Serious and fatal adverse reactions including "gasping syndrome" can occur in neonates and infants treated with benzyl alcohol-preserved drugs, including INCRELEX. The "gasping syndrome" is characterized by central nervous system depression, metabolic acidosis, and gasping respirations.
Use of INCRELEX in infants is not recommended [see Use in Specific Populations 8.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hypoglycemia [see Warnings and Precautions (5.1)].
- Hypersensitivity and Allergic Reactions, including Anaphylaxis [see Warnings and Precautions (5.2)]
- Intracranial hypertension (IH) [see Warnings and Precautions (5.3)]
- Tonsillar and Adenoidal Hypertrophy and related complications [see Warnings and Precautions (5.4)]
- Slipped Capital Femoral Epiphysis [see Warnings and Precautions (5.5)]
- Progression of Preexisting Scoliosis [see Warnings and Precautions (5.6)]
- Malignant Neoplasia [see Warnings and Precautions (5.7)]
- Benzyl Alcohol [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical studies of 71 subjects with Primary IGFD treated for a mean duration of 3.9 years and representing 274 subject-years, no subjects withdrew from any clinical study because of adverse reactions. Adverse reactions to INCRELEX treatment that occurred in 5% or more of these study participants are listed below by organ class.
Metabolism and Nutrition Disorders: hypoglycemia
General Disorders and Administrative Site Conditions: lipohypertrophy, bruising
Infections and Infestations: otitis media, serous otitis media
Respiratory, Thoracic and Mediastinal Disorders: snoring, tonsillar hypertrophy
Nervous System Disorders: headache, dizziness, convulsions
Gastrointestinal Disorders: vomiting
Ear and Labyrinth Disorders: hypoacusis, fluid in middle ear, ear pain, abnormal tympanometry
Cardiac Disorders: cardiac murmur
Musculoskeletal and Connective Tissue Disorders: arthralgia, pain in extremity
Blood and Lymphatic System Disorders: thymus hypertrophy
Surgical and Medical Procedures: ear tube insertion
Hypoglycemia was reported by 30 subjects (42%) at least once during their course of therapy. Most cases of hypoglycemia were mild or moderate in severity. Five subjects had severe hypoglycemia (requiring assistance and treatment) on one or more occasions and 4 subjects experienced hypoglycemic seizures/loss of consciousness on one or more occasions. Of the 30 subjects reporting hypoglycemia, 14 (47%) had a history of hypoglycemia prior to treatment. The frequency of hypoglycemia was highest in the first month of treatment, and episodes were more frequent in younger children. Symptomatic hypoglycemia was generally avoided when a meal or snack was consumed either shortly (i.e., 20 minutes) before or after the administration of INCRELEX.
Tonsillar hypertrophy was noted in 11 (15%) subjects in the first 1 to 2 years of therapy with lesser tonsillar growth in subsequent years. Tonsillectomy or tonsillectomy/adenoidectomy was performed in 7 subjects; 3 of these had obstructive sleep apnea, which resolved after the procedure in all three cases.
Intracranial hypertension occurred in three subjects. In two subjects the events resolved without interruption of INCRELEX treatment. INCRELEX treatment was discontinued in the third subject and resumed later at a lower dose without recurrence.
Mild elevations in the serum AST and LDH were found in a significant proportion of patients before and during treatment. Rise in levels of these serum enzymes did not lead to treatment discontinuation. ALT elevations were occasionally noted during treatment.
Renal and splenic lengths (measured by ultrasound) increased rapidly on INCRELEX treatment during the first years of therapy. This lengthening slowed down subsequently; though in some patients, renal and/or splenic length reached or surpassed the 95th percentile. Renal function (as defined by serum creatinine and calculated creatinine clearance) was normal in all patients, irrespective of renal growth.
Elevations in cholesterol and triglycerides to above the upper limit of normal were observed before and during treatment.
Echocardiographic evidence of cardiomegaly/valvulopathy was observed in a few individuals without associated clinical symptoms. The relation of these cardiac changes to drug treatment cannot be assessed due to underlying disease and the lack of a control group.
Thickening of the soft tissues of the face was observed in several patients and should be monitored during INCRELEX treatment.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to INCRELEX with the incidence of antibodies to other products may be misleading.
Anti-IGF-1 antibodies were present at one or more of the periodic assessments in 14 of 23 children with Primary IGFD treated for 2 years. However, no clinical consequences of these antibodies were observed (e.g., attenuation of growth).
6.3 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of INCRELEX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Systemic hypersensitivity: anaphylaxis, generalized urticaria, angioedema, dyspnea
In the post-marketing setting, the frequency of cases indicative of anaphylaxis was estimated to be 0.3%. Symptoms included hives, angioedema, and dyspnea, and some patients required hospitalization. Upon re-administration, symptoms did not re-occur in all patients.
Local allergic reactions at the injection site: pruritus, urticaria
Skin and Subcutaneous Tissue Disorders: alopecia, hair texture abnormal
General Disorders and Administrative Site Conditions: injection site reactions (e.g. erythema, pain, hematoma, hemorrhage, induration, rash, swelling)
Musculoskeletal and Connective Tissue Disorders: osteonecrosis/avascular necrosis (occasionally associated with slipped capital femoral epiphysis)
Neoplasms Benign, Malignant and Unspecified (including cysts and polyps)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on INCRELEX use in pregnant women. Exposure to INCRELEX during pregnancy is unlikely because the drug is not indicated for use after epiphyseal closure. In animal reproduction studies, there were no observed embryo-fetal development abnormalities with intravenous administration of INCRELEX to pregnant rats and rabbits during fetal organogenesis given at exposures up to 11 and 3 times the maximum recommended human dose (MRHD) of 0.24 mg/kg/day based on body surface area (BSA), respectively (see Data).
The estimated background risk of birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Studies to assess embryo-fetal toxicity evaluated the effects of INCRELEX during organogenesis in Sprague Dawley rats given 1, 4, and 16 mg/kg/day and in New Zealand White rabbits given 0.125, 0.5, and 2 mg/kg/day, administered intravenously. There were no observed embryo-fetal developmental abnormalities in rats given up to 16 mg/kg/day (11 times the MRHD based on BSA comparison). In the rabbit study, the NOAEL for fetal toxicity was 0.5 mg/kg/day (approximately equivalent to the MRHD based on BSA) due to an increase in fetal death at 2 mg/kg. INCRELEX displayed no teratogenicity or maternal toxicity in rabbits given up to 2 mg/kg (3 times the MRHD based on BSA).
8.2 Lactation
Risk Summary
There is no information available on the presence of mecasermin in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for INCRELEX and any potential adverse effects on the breast-fed child from INCRELEX or from the underlying maternal condition.
8.4 Pediatric Use
Toxicity (Gasping Syndrome) with Benzyl Alcohol
Serious adverse reactions including fatal reactions and the "gasping syndrome" occurred in premature neonates and infants in the intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 mg/kg/day to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 mmol/L to 1.378 mmol/L). INCRELEX contains 9 mg/mL benzyl alcohol as a preservative.
Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. Use of INCRELEX in infants is not recommended [see Warnings and Precautions (5.8)].
Safety and effectiveness in pediatric patients below the age of 2 years of age have not been established.
8.5 Geriatric Use
The safety and effectiveness of INCRELEX in patients aged 65 and over has not been established.
8.6 Renal Impairment
No Studies have been conducted in Primary IGFD children or adult subjects with renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No studies have been conducted in Primary IGFD children or adult subjects with hepatic impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Treatment of acute overdose should be directed at reversing hypoglycemia. Oral glucose or food should be consumed. If the overdose results in loss of consciousness, intravenous glucose or parenteral glucagon may be required to reverse the hypoglycemic effects.
A small number of overdose cases have been reported in the post-marketing experience. In one case of acute overdose, a 3-year old patient experienced hypoglycemia after receiving one 4 mg dose of INCRELEX (a 10-fold increase beyond the prescribed dose). The event resolved following treatment with IV glucose.
Long term overdosage with INCRELEX may result in signs and symptoms of acromegaly.
-
11 DESCRIPTION
INCRELEX (mecasermin) injection contains human insulin-like growth factor-1 (rhIGF-1) produced by recombinant DNA technology. IGF-1 consists of 70 amino acids in a single chain with three intramolecular disulfide bridges and a molecular weight of 7649 daltons. The amino acid sequence of the product is identical to that of endogenous human IGF-1. The rhIGF-1 protein is synthesized in bacteria (E. coli) that have been modified by the addition of the gene for human IGF-1.
INCRELEX is a sterile, aqueous, clear and colorless solution intended for subcutaneous injection. Each multi-dose vial of INCRELEX contains 10 mg per mL mecasermin, 9 mg per mL benzyl alcohol, 5.84 mg per mL sodium chloride, 2 mg per mL polysorbate 20, and 0.05M acetate at a pH of approximately 5.4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Insulin-like growth factor-1 (IGF-1) is a key hormonal mediator on statural growth. Under normal circumstances, growth hormone (GH) binds to its receptor in the liver, and other tissues, and stimulates the synthesis/secretion of IGF-1. In target tissues, the Type 1 IGF-1 receptor, which is homologous to the insulin receptor, is activated by IGF-1, leading to intracellular signaling which stimulates multiple processes resulting in statural growth. The metabolic actions of IGF-1 are in part directed at stimulating the uptake of glucose, fatty acids, and amino acids so that metabolism supports growing tissues.
12.2 Pharmacodynamics
The following actions have been demonstrated for endogenous human IGF-1:
Tissue Growth – 1) Skeletal growth occurs at the cartilage growth plates of the epiphyses of bones where stem cells divide to produce new cartilage cells or chondrocytes. The growth of chondrocytes is under the control of IGF-1 and GH. The chondrocytes become calcified so that new bone is formed allowing the length of the bones to increase. This results in skeletal growth until the cartilage growth plates fuse at the end of puberty. 2) Cell growth: IGF-1 receptors are present on most types of cells and tissues. IGF-1 has mitogenic activities that lead to an increased number of cells in the body. 3) Organ growth: Treatment of IGF-1 deficient rats with rhIGF-1 results in whole body and organ growth.
12.3 Pharmacokinetics
Absorption – The absolute bioavailability of rhIGF-1 after subcutaneous administration in healthy subjects is estimated to be close to 100%. However, the absolute bioavailability of INCRELEX given subcutaneously to subjects with primary insulin-like growth factor-1 deficiency (Primary IGFD) has not been determined.
Distribution – In blood, IGF-1 is bound to six IGF binding proteins, with > 80% bound as a complex with IGFBP-3 and an acid-labile subunit. IGFBP-3 is greatly reduced in subjects with severe Primary IGFD, resulting in increased clearance of IGF-1 in these subjects relative to healthy subjects. The total IGF-1 volume of distribution after subcutaneous administration in subjects with severe Primary IGFD is estimated to be 0.257 (± 0.073) L/kg at an INCRELEX dose of 0.045 mg/kg, and is estimated to increase as the dose of INCRELEX increases.
Elimination – IGF-1 is metabolized by both liver and kidney. The mean terminal t1/2 after single subcutaneous administration of 0.12 mg/kg INCRELEX in pediatric subjects with severe Primary IGFD is estimated to be 5.8 hours. Clearance of INCRELEX is inversely proportional to IGF binding protein-3 (IGFBP-3) levels. CL/F is estimated to be 0.04 L/hr/kg at 0.5 micrograms/mL of IGFBP-3, and 0.01 L/hr/kg at 3 micrograms/mL IGFBP-3; the latter is the median IGFBP-3 in subjects with normal IGF-1 serum levels.
Gender – In children with Primary IGFD there were no apparent differences between males and females in the pharmacokinetics of INCRELEX.
Race –The effect of race on pharmacokinetics of INCRELEX has not been studied.
Summary of INCRELEX Single-Dose Pharmacokinetic Parameters in Children with Severe Primary IGFD (0.12 mg/kg, SC) Cmax
(ng/mL)Tmax
(hr)AUC0-8
(hr*ng/mL)t1/2
(hr)Vd/F
(L/kg)CL/F
(L/hr/kg)n 3 3 3 3 12* 12* Cmax = maximum concentration; Tmax = time of maximum concentration; AUC0-8 = area under the curve; t1/2 = half-life; Vd/F = apparent volume of distribution; CL/F = apparent systemic clearance; SC = subcutaneous injection; CV% = coefficient of variation in %.
Male/female data combined, ages 12 to 22 years.
PK parameters based on baseline adjusted plasma concentrations.- * Data represents 3 subjects each at doses 0.015, 0.03, 0.06, and 0.12 mg/kg SC.
Mean 234 2 2932 5.8 0.257 0.0424 CV% 23 0 50 64 28 38 Mean Total IGF-1 Concentration after a Single Subcutaneous Dose of INCRELEX in Children with Severe Primary IGFD (0.06 mg/kg and 0.12 mg/kg, n = 3 per group) 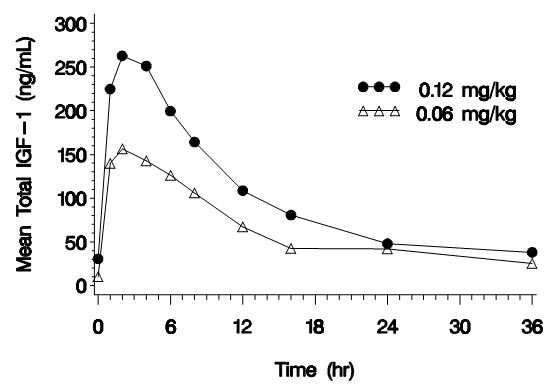
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: INCRELEX was tumorigenic in rats in a study using doses of 0, 0.25, 1, 4, and 10 mg/kg/day by subcutaneous injection for up to 2 years. The incidence of adrenal medullary hyperplasia and pheochromocytoma increased in male rats given ≥1 mg/kg/day (below clinical exposure at the maximum recommended human dose [MRHD] based on AUC) and in female rats at all dose levels (below clinical exposure at the MRHD based on AUC). The incidence of keratoacanthoma in the skin increased in male rats given 4 and 10 mg/kg/day (approximately the clinical exposure at the MRHD based on AUC). The incidence of mammary gland carcinoma in male rats increased in animals treated with 10 mg/kg/day (3 times the MRHD based on AUC). Only doses that exceeded the maximum tolerated dose (MTD) (based on excess mortality secondary to IGF-1 induced hypoglycemia) caused skin and mammary tumors.
-
14 CLINICAL STUDIES
14.1 Effects of INCRELEX Treatment in Children with Severe Primary Insulin-like Growth Factor-1 Deficiency (Severe Primary IGFD)
Five clinical studies (four open-label and one double-blind, placebo-controlled), with subcutaneous doses of INCRELEX generally ranging from 0.06 to 0.12 mg/kg (60 to 120 micrograms/kg) administered twice daily, were conducted in 71 pediatric subjects with severe Primary IGFD. Patients were enrolled in the trials on the basis of extreme short stature, slow growth rates, low IGF-1 serum concentrations, and normal growth hormone secretion. Data from these 5 clinical studies were pooled for a global efficacy and safety analysis. Baseline characteristics for the patients evaluated in the primary and secondary efficacy analyses were (mean, SD): chronological age (years): 6.7 ± 3.8; height (cm): 84.8 ± 15.3 cm; height standard deviation score (SDS): -6.7 ± 1.8; height velocity (cm/yr): 2.8 ± 1.8; height velocity SDS: -3.3 ± 1.7; IGF-1 (ng/mL): 21.6 ± 20.6; IGF-1 SDS: -4.3 ± 1.6; and bone age (years): 4.2 ± 2.8. Sixty-one subjects had at least one year of treatment. Fifty-three (87%) had Laron Syndrome; 7 (11%) had GH gene deletion, and 1 (2%) had neutralizing antibodies to GH. Thirty-seven (61%) of the subjects were male; forty-eight (79%) were Caucasian. Fifty-six (92%) of the subjects were pre-pubertal at baseline.
Annual results for height velocity, height velocity SDS, and height SDS are shown in Table 1. Pre-treatment height velocity data were available for 58 subjects. The height velocities at a given year of treatment were compared by paired t-tests to the pre-treatment height velocities of the same subjects completing that treatment year.
Table 1: Annual Height Results by Number of Years Treated with INCRELEX Pre-Tx Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Year 7 Year 8 Pre-Tx = Pre-treatment; SD = Standard Deviation; SDS = Standard Deviation Score
- * P-values for comparison versus pre-treatment values are computed using paired t-tests.
Height Velocity (cm/yr) N 58 58 48 38 23 21 20 16 13 Mean (SD) 2.8 (1.8) 8.0 (2.2) 5.8 (1.5) 5.5 (1.8) 4.7 (1.6) 4.7 (1.6) 4.8 (1.5) 4.6 (1.5) 4.3 (1.1) Mean (SD) for change from pre-treatment +5.2 (2.6) +2.9 (2.4) +2.3 (2.4) +1.5 (2.2) +1.5 (1.8) +1.5 (1.7) +1.0 (2.1) +0.7 (2.5) P-value for change from pre-treatment * <0.0001 <0.0001 <0.0001 0.0045 0.0015 0.0009 0.0897 0.3059 Height Velocity SDS N 58 58 47 37 22 19 18 15 11 Mean (SD) -3.3 (1.7) 1.9 (3.0) -0.2 (1.6) -0.2 (2.0) -0.7 (2.1) -0.6 (2.1) -0.4 (1.4) -0.4 (1.9) -0.4 (1.9) Mean (SD) for change from pre-treatment +5.2 (3.1) +3.1 (2.3) +2.9 (2.3) +2.2 (2.2) +2.5 (2.2) +2.7 (1.7) +2.5 (2.1) +2.7 (2.8) Height SDS N 61 61 51 40 24 21 20 16 13 Mean (SD) -6.7 (1.8) -5.9 (1.8) -5.6 (1.8) -5.4 (1.8) -5.5 (1.9) -5.6 (1.8) -5.4 (1.8) -5.2 (2.0) -5.2 (2.0) Mean (SD) for change from pre-treatment +0.8 (0.5) +1.2 (0.8) +1.4 (1.1) +1.3 (1.2) +1.4 (1.3) +1.4 (1.2) +1.4 (1.1) +1.5 (1.1) Forty-nine subjects were included in an analysis of the effects of INCRELEX on bone age advancement. The mean ± SD change in chronological age was 4.9 ± 3.4 years and the mean ± SD change in bone age was 5.3 ± 3.4 years.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC-15054-1040-5 INCRELEX is supplied as a 10 mg per mL sterile solution in multiple dose glass vials (40 mg per vial). Before Opening – Vials of INCRELEX are stable when refrigerated [2° to 8°C (35° to 46°F)]. Avoid freezing the vials of INCRELEX. Protect from direct light. Expiration dates are stated on the labels.
After Opening – Vials of INCRELEX are stable for 30 days after initial vial entry when stored at 2° to 8°C (35° to 46°F). Avoid freezing the vials of INCRELEX. Protect from direct light.
Vial contents should be clear without particulate matter. If the solution is cloudy or contains particulate matter, the contents must not be injected. INCRELEX should not be used after its expiration date. Keep refrigerated and use within 30 days of initial vial entry. Remaining unused material should be discarded.
-
17 PATIENT COUNSELING INFORMATION
Advise patients and/or caregivers to read the FDA-approved patient labeling (Patient Information) and Instructions for Use.
Counsel patients and/or parents that there have been occurrences of malignant neoplasia observed among pediatric patients who received treatment with INCRELEX. Instruct patients and/or parents to monitor for development of any new growth or symptoms of cancer and to report it immediately.
Instruct patients and/or caregivers in the proper administration of INCRELEX. Give INCRELEX shortly before or after (20 minutes on either side of) a meal or snack. Do not give INCRELEX when the meal or snack is omitted. Do not increase the dose of INCRELEX to make up for one or more omitted doses. INCRELEX therapy will be initiated at a low dose and the dose should be increased only if no hypoglycemia episodes have occurred after at least 7 days of dosing. Educate patients and caregivers on how to recognize the signs and symptoms of hypoglycemia.
Educate patients and caregivers on the identification of signs and symptoms of serious allergic reactions to INCRELEX and the need to seek prompt medical contact should such a reaction occur. Instruct patients and caregivers to discontinue INCRELEX if a reaction occurs.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 12/2019 PATIENT INFORMATION
INCRELEX® (EENK-RUH-LEX)
(mecasermin) injection
for subcutaneous useRead the Patient Information that comes with INCRELEX before your child starts receiving INCRELEX and each time your child gets a refill. There may be new information. This Patient Information leaflet does not take the place of talking with your child's doctor about your child's medical condition or treatment.
What is INCRELEX?
- INCRELEX is a liquid that contains man-made insulin-like growth factor-1 (IGF-1). INCRELEX is used to treat children who are very short for their age because their bodies do not make enough IGF-1. This condition is called primary IGF-1 deficiency.
- IGF-1 should not be used instead of growth hormone.
- It is not known if INCRELEX is safe and effective in children under 2 years of age.
Who should not receive INCRELEX?
Your child should not receive INCRELEX if your child:
- is allergic to mecasermin or any of the ingredients in INCRELEX. See the end of this Patient Information leaflet for a complete list of ingredients in INCRELEX. Check with your child's doctor if you are not sure.
- has finished growing (the bone growth plates are closed).
- has any cancerous tumors or growths; or has a history of cancer or a condition that increases the risk of cancer.
Your child should never receive INCRELEX through a vein.
What should I tell my child's doctor before my child starts receiving INCRELEX?
Tell your child's doctor about all of your child's medical conditions, including if your child:
- has diabetes.
- has a curved spine (scoliosis).
- is pregnant or you think your child might be pregnant. It is not known if INCRELEX can harm an unborn baby.
- is breastfeeding or plans to breastfeed. Talk to your child's doctor about the best way for your child to feed her baby if she receives INCRELEX.
Tell your child's doctor about all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your child's doctor if your child takes insulin or other anti-diabetes medicines. A change in dose may be needed for these medicines.
How should my child receive INCRELEX?
- Use INCRELEX exactly as prescribed for your child. Your child's doctor or nurse should teach you how to inject INCRELEX. Do not give your child INCRELEX unless you understand all of the instructions.
- See the "Instructions for Use" at the end of this Patient Information leaflet.
- Inject INCRELEX under your child's skin 20 minutes before or 20 minutes after a meal or snack.
- Skip your child's dose of INCRELEX if your child cannot eat for any reason. Do not give your child an extra dose of INCRELEX or increase the amount of your child's dose to make up for a missed dose.
What are the possible side effects of INCRELEX?
INCRELEX may cause serious side effects, including:
- Low blood sugar (hypoglycemia). INCRELEX may lower blood sugar levels. It is important to only give your child INCRELEX 20 minutes before or 20 minutes after a meal or snack to reduce the chances of low blood sugar. Do not give your child INCRELEX if your child cannot eat. Signs of low blood sugar include:
- dizziness
- tiredness
- restlessness
- hunger
- irritability
- trouble concentrating
- sweating
- nausea
- fast or irregular heartbeat
Severe hypoglycemia may cause unconsciousness, seizures, or death. If your child receives INCRELEX, they should avoid participating in high risk activities (e.g. driving, exercise, etc.) within 2 to 3 hours after the INCRELEX injection, especially at the beginning of INCRELEX treatment.
Before beginning treatment with INCRELEX your child's doctor or nurse will explain to you how to treat hypoglycemia. Your child should always have a source of sugar such as orange juice, glucose gel, candy, or milk available in case symptoms of hypoglycemia happen. For severe hypoglycemia, if your child is not responsive and cannot drink sugar-containing fluids, you should get emergency medical help for your child right away. Your doctor or nurse will show you how to give the injection.
-
Allergic reactions. See "Who should not receive INCRELEX?" Allergic reactions are a serious but common side effect of INCRELEX. Your child may have a mild or serious allergic reaction with INCRELEX. Call your child's doctor right away if your child gets a rash or hives. Hives, also known as urticaria, appear as a raised, itchy skin reaction. Hives appear pale in the middle with a red rim around it. Hives generally appear minutes to hours after the injection and may sometimes occur at many places on the skin. Stop taking INCRELEX and get medical help right away if your child has trouble breathing or goes into shock, with symptoms like dizziness, pale, clammy skin or passing out.
INCRELEX can also cause reactions at the injection site including:- loss of fat (lipoatrophy)
- increase of fat (lipohypertrophy)
- pain, redness, or bruising
- Increased pressure in the brain (intracranial hypertension). INCRELEX, like growth hormone, can sometimes cause a temporary increase in pressure within the brain. The symptoms of intracranial hypertension can include headache, nausea and vomiting. Tell your doctor if your child has a headache with vomiting. Your doctor can then check to see if intracranial hypertension is present. If it is present, your child's doctor may decide to temporarily reduce or stop INCRELEX therapy.
- Enlarged tonsils. Some signs of enlarged tonsils include: snoring, difficulty breathing, sleep apnea (a condition where breathing stops briefly during sleep), or fluid in the middle-ear. Sleep apnea can cause excessive daytime sleepiness. Call your child's doctor if these symptoms bother your child. Your child's doctor should do regular exams to check your child's tonsils.
- A bone problem called slipped capital femoral epiphysis. This happens when the top of the upper leg (femur) slips apart. Get medical help for your child right away if your child develops a limp or has hip or knee pain.
- Worsened scoliosis (caused by rapid growth). If your child has scoliosis, your child will need to be checked often for an increase in the curve of the spine.
- Tumor Growths. Several cases of cancerous tumors have been observed in pediatric patients who received treatment with INCRELEX. Tell your doctor immediately if your child develops any new growths or symptoms of cancer.
- Benzyl alcohol toxicity. Benzyl alcohol is a preservative in INCRELEX. Benzyl alcohol can cause serious side effects, including death in infants who have received medicines that contain the benzyl alcohol preservative.
The most common side effects of INCRELEX include: hypoglycemia (low blood sugar), injection site reactions, allergic reactions and enlarged tonsils.
Call your child's doctor if your child has any side effects that bothers them or that does not go away.
These are not all the possible side effects of INCRELEX. For more information, ask your child's doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store INCRELEX?
- Before Opening: Store new, unopened vials of INCRELEX in the refrigerator between 35°F to 46°F (2°C to 8°C).
- After Opening: Store opened vials of INCRELEX in the refrigerator between 35°F to 46°F (2°C to 8°C) for 30 days after you start using the vial. Throw away any unused INCRELEX after 30 days.
- Do not freeze INCRELEX. If a vial freezes, throw it away.
- Keep INCRELEX out of direct light.
- Do not use INCRELEX after the expiration date printed on the label.
Keep INCRELEX and all medicines out of the reach of children.
General information about the safe and effective use of INCRELEX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not give INCRELEX to your child for a condition for which it was not prescribed. Do not give INCRELEX to a person other than your child. It may harm them.
This Patient Information leaflet summarizes the most important information about INCRELEX. If you would like more information, talk to your child's doctor. You can also ask your child's doctor or pharmacist for information about INCRELEX that is written for health professionals.
What are the ingredients in INCRELEX?
Active ingredient: mecasermin
Inactive ingredients: benzyl alcohol, sodium chloride, polysorbate 20, and acetate.
Manufactured for: Ipsen Biopharmaceuticals, Inc.
INCRELEX is a registered trademark of Ipsen Biopharmaceuticals, Inc.
© 2019. All rights reserved.
For more information, go to www.Increlex.com or call 855-463-5127. -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
INCRELEX® (EENK-RUH-LEX)
(mecasermin) injection
for subcutaneous useRead this Instructions for Use before you start using INCRELEX and each time you get a refill. There may be new information. This information does not take the place of talking to your child's doctor about your child's medical condition or treatment.
Do not share your child's needles and syringes with another person. Your child may give another person an infection or your child could get an infection from them.
Important:
- Inject INCRELEX exactly as your child's doctor or nurse has shown you.
- Follow your doctor's instructions for the type of syringe and needle to use to prepare and inject your child's dose of INCRELEX.
- Always use a new, unopened needle and syringe for each injection.
- Only use single-use, disposable needles and syringes. Never reuse disposable needles and syringes.
- Throw away used needles and syringes in a puncture-resistant, disposable sharps container as soon as you finish giving the injection. See step 5 "How should I throw away (dispose of) used needles and syringes?" at the end of these instructions.
Supplies needed to give the injection:
- 1 vial of INCRELEX
- 1 alcohol swab
- 1 gauze or cotton ball
- alcohol (to clean the skin at the injection site)
- 1 sharps container for throwing away (disposing of) used needles and syringes. See step 5 "How should I throw away (dispose of) used needles and syringes?" at the end of these instructions.
Preparing the Dose:
- Wash your hands before getting INCRELEX ready for your child's injection.
- Check the liquid to make sure it is clear and colorless. Do not use if it is cloudy or if you see particles.
- Check the expiration date printed on the label of the vial. Do not use INCRELEX if the expiration date has passed.
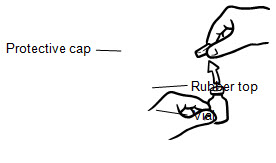
- If you are using a new vial, remove the protective cap. Do not remove the rubber top (see Figure 1).
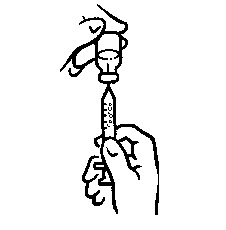
- Wipe the rubber top on the vial with an alcohol swab (see Figure 2).
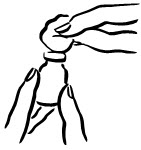
- Before putting the needle into the vial, pull back on plunger to draw air into the syringe equal to the INCRELEX dose. Put the needle through the rubber top of the vial and push the plunger to inject air into the vial (see Figure 3).
- Leave the syringe in the vial and turn both upside down. Hold the syringe and vial firmly (see Figure 4).
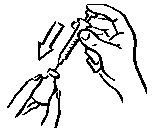
- Make sure the tip of the needle is in the liquid (see Figure 5). Pull the plunger to withdraw the correct dose into the syringe (see Figure 6).
- Before you take the needle out of the vial, check the syringe for air bubbles. If bubbles are in the syringe, hold the vial and syringe with needle straight up and tap the side of the syringe until the bubbles float to the top. Push the bubbles out with the plunger and draw liquid back in until you have the correct dose (see Figure 7).
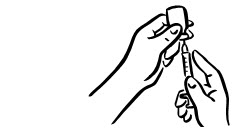
- Remove the needle from the vial. Do not let the needle touch anything. You are now ready to inject (see Figure 8).
Injecting the Dose:
Inject INCRELEX exactly as your child's doctor or nurse has shown you.
Do not give the INCRELEX injection if your child is unable to eat within 20 minutes before or after the injection.
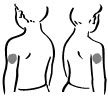
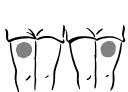
- 1. Choose an injection site – upper arm, upper leg (thigh), buttocks, or stomach area (abdomen) (see below). The injection site should be changed (rotated) for each injection.
Upper arm Thigh Buttock Abdomen - 2.
Use alcohol to clean the skin where you are going to inject your child. The injection site should be dry before you inject.
Do not fan or blow on the cleaned skin.
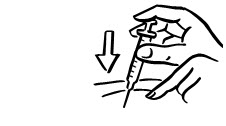
Do not touch the injection site again before giving the injection. - 3. Lightly pinch the skin. Insert the needle into the pinched skin as instructed by your child's doctor or nurse (see Figure A). Release the pinched skin.
- 4. Slowly push the plunger of the syringe all the way in to make sure you have injected all of the liquid. Pull the needle straight out and gently press on the injection site with gauze or a cotton ball for a few seconds. Do not rub the injection site (see Figure B).
- 5.
How should I throw away (dispose of) used needles and syringes?
- Put used needles and syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- Do not try to touch the needle.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how to throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- For the safety and health of you and others, needles and used syringes must never be re-used.
- The used alcohol swabs, cotton balls, and gauze may be placed in your household trash.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Always keep the sharps disposal container out of the reach of children.
How should I store INCRELEX?
- Before Opening: Store new, unopened vials of INCRELEX in the refrigerator between 35°F to 46°F (2°C to 8°C).
- After Opening: Store opened vials of INCRELEX in the refrigerator between 35°F to 46°F (2°C to 8°C) for 30 days after you start using the vial. Throw away any unused INCRELEX after 30 days.
- Do not freeze INCRELEX. If a vial freezes, throw it away.
- Keep INCRELEX out of direct light.
- Do not use INCRELEX after the expiration date printed on the label.
Keep INCRELEX and all medicines out of reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
For additional information, call 855-463-5127.
Manufactured for: Ipsen Biopharmaceuticals, Inc.
Cambridge, MA 02142USAwww.ipsenus.com
Revised: December 2019
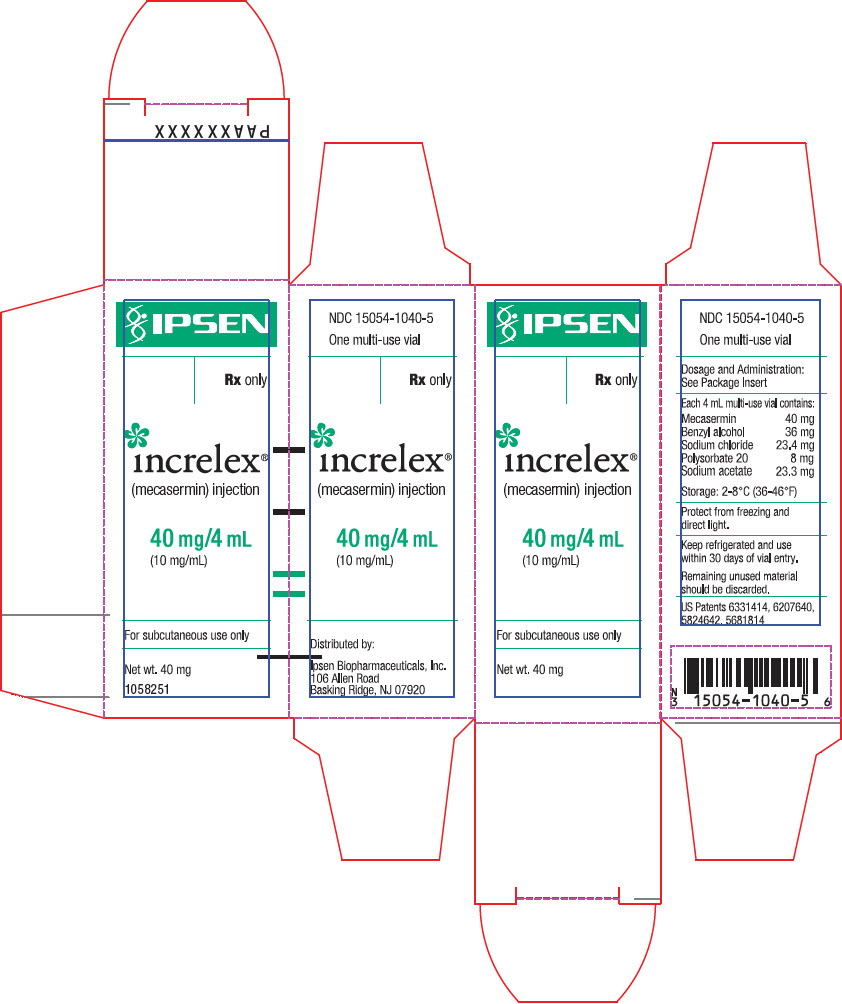
- PRINCIPAL DISPLAY PANEL - 40 mg Vial Carton
-
INGREDIENTS AND APPEARANCE
INCRELEX
mecasermin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-1040 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mecasermin (UNII: 7GR9I2683O) (mecasermin - UNII:7GR9I2683O) mecasermin 40 mg in 4 mL Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) 36 mg in 4 mL sodium chloride (UNII: 451W47IQ8X) 23.4 mg in 4 mL polysorbate 20 (UNII: 7T1F30V5YH) 8 mg in 4 mL sodium acetate (UNII: 4550K0SC9B) 23.3 mg in 4 mL acetic acid (UNII: Q40Q9N063P) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-1040-5 1 in 1 CARTON 01/03/2006 1 4 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021839 01/03/2006 Labeler - Ipsen Biopharmaceuticals, Inc. (118461578) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 030606222 MANUFACTURE(15054-1040)
Trademark Results [Increlex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INCRELEX 78696484 3345436 Live/Registered |
IPSEN BIOPHARMACEUTICALS, INC. 2005-08-19 |
 INCRELEX 78423297 3149571 Live/Registered |
IPSEN BIOPHARMACEUTICALS, INC. 2004-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.