PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE solution
Promethazine Hydrochloride and Dextromethorphan Hydrobromide by
Drug Labeling and Warnings
Promethazine Hydrochloride and Dextromethorphan Hydrobromide by is a Prescription medication manufactured, distributed, or labeled by Tris Pharma Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Each 5 mL (one teaspoonful), for oral administration contains: dextromethorphan hydrobromide 15 mg; promethazine hydrochloride 6.25 mg. Alcohol 8%(v/v).
Inactive Ingredients: anhydrous citric acid, ascorbic acid, edetate disodium, FD&C yellow No. 6, methylparaben, natural and artificial lemon mint flavor, propylene glycol, propylparaben, purified water, sodium benzoate, sodium citrate anhydrous, saccharin sodium, and sucrose.
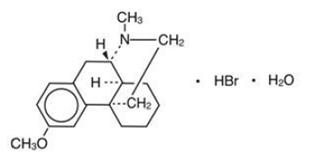
Dextromethorphan hydrobromide is a salt of the methyl ether of the dextrorotatory isomer of levorphanol, a narcotic analgesic. It is chemically designated as 3-methoxy-17-methyl-9α, 13α, 14α-morphinan hydrobromide monohydrate. Dextromethorphan hydrobromide occurs as white crystals sparingly soluble in water and freely soluble in alcohol. It has a molecular weight of 370.32, a molecular formula of C18H25NOHBrH2O, and the following structural formula:
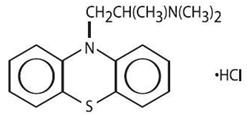
Promethazine is a racemic compound. Promethazine hydrochloride, a phenothiazine derivative, is chemically designated as 10H-Phenothiazine-10-ethanamine, N,N, α-trimethyl-, monohydrochloride.
Promethazine hydrochloride occurs as a white to faint yellow, practically odorless, crystalline powder which slowly oxidizes and turns blue on prolonged exposure to air. It is soluble in water and freely soluble in alcohol. It has a molecular weight of 320.88, a molecular formula of C17H20N2SHCl, and the following structural formula:
-
CLINICAL PHARMACOLOGY
Dextromethorphan: Dextromethorphan is an antitussive agent and, unlike the isomeric levorphanol, it has no analgesic or addictive properties.
The drug acts centrally and elevates the threshold for coughing. It is about equal to codeine in depressing the cough reflex. In therapeutic dosage dextromethorphan does not inhibit ciliary activity.
Dextromethorphan is rapidly absorbed from the gastrointestinal tract and exerts its effect in 15 to 30 minutes. The duration of action after oral administration is approximately three to six hours. Dextromethorphan is metabolized primarily by liver enzymes undergoing O-demethylation, N-demethylation, and partial conjugation with glucuronic acid and sulfate. In humans, (+)-3-hydroxy-N-methyl-morphinan, (+)-3-hydroxymorphinan, and traces of unmetabolized drug were found in urine after oral administration.
Promethazine: Promethazine is a phenothiazine derivative which differs structurally from the antipsychotic phenothiazines by the presence of a branched side chain and no ring substitution. It is thought that this configuration is responsible for its relative lack (1/10 that of chlorpromazine) of dopamine antagonist properties.
Promethazine is an H1 receptor blocking agent. In addition to its antihistaminic action, it provides clinically useful sedative and antiemetic effects.
Promethazine is well absorbed from the gastrointestinal tract. Clinical effects are apparent within 20 minutes after oral administration and generally last four to six hours, although they may persist as long as 12 hours. Promethazine is metabolized by the liver to a variety of compounds; the sulfoxides of promethazine and N-demethylpromethazine are the predominant metabolites appearing in the urine.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution is contraindicated for use in pediatric patients less than two years of age.
Dextromethorphan should not be used in patients receiving a monoamine oxidase inhibitor (MAOI) (see PRECAUTIONS, Drug Interactions).
Promethazine is contraindicated in comatose states, and in individuals known to be hypersensitive or to have had an idiosyncratic reaction to promethazine or to other phenothiazines.
Antihistamines are contraindicated for use in the treatment of lower respiratory tract symptoms, including asthma.
-
WARNINGS
WARNING
PROMETHAZINE HYDROCHLORIDE SHOULD NOT BE USED IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE BECAUSE OF THE POTENTIAL FOR FATAL RESPIRATORY DEPRESSION.
POSTMARKETING CASES OF RESPIRATORY DEPRESSION, INCLUDING FATALITIES, HAVE BEEN REPORTED WITH USE OF PROMETHAZINE HYDROCHLORIDE IN PEDIATRIC PATIENTS LESS THAN 2 YEARS OF AGE. A WIDE RANGE OF WEIGHT-BASED DOSES OF PROMETHAZINE HYDROCHLORIDE HAVE RESULTED IN RESPIRATORY DEPRESSION IN THESE PATIENTS.
CAUTION SHOULD BE EXERCISED WHEN ADMINISTERING PROMETHAZINE HYDROCHLORIDE TO PEDIATRIC PATIENTS 2 YEARS OF AGE AND OLDER. IT IS RECOMMENDED THAT THE LOWEST EFFECTIVE DOSE OF PROMETHAZINE HYDROCHLORIDE BE USED IN PEDIATRIC PATIENTS 2 YEARS OF AGE AND OLDER AND CONCOMITANT ADMINISTRATION OF OTHER DRUGS WITH RESPIRATORY DEPRESSANT EFFECTS BE AVOIDED.
Dextromethorphan: Administration of dextromethorphan may be accompanied by histamine release and should be used with caution in atopic children.
Promethazine:
CNS Depression
Promethazine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. The impairment may be amplified by concomitant use of other central-nervous-system depressants such as alcohol, sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers; therefore such agents should either be eliminated or given in reduced dosage in the presence of promethazine HCl (see PRECAUTIONS-Information for Patients and Drug Interactions).
Respiratory Depression
Promethazine may lead to potentially fatal respiratory depression.
Use of promethazine in patients with compromised respiratory function (e.g., COPD, sleep apnea) should be avoided.
Lower Seizure Threshold
Promethazine may lower seizure threshold. It should be used with caution in persons with seizure disorders or in persons who are using concomitant medications, such as narcotics or local anesthetics, which may also affect seizure threshold.
Bone-Marrow Depression
Promethazine should be used with caution in patients with bone-marrow depression. Leukopenia and agranulocytosis have been reported, usually when promethazine HCl has been used in association with other known marrow-toxic agents.
Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with promethazine HCl alone or in combination with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g. pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of promethazine HCl, antipsychotic drugs, if any, and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
Since recurrences of NMS have been reported with phenothiazines, the reintroduction of promethazine HCl should be carefully considered.
Use In Pediatric Patients
PROMETHAZINE PRODUCTS ARE CONTRAINDICATED FOR USE IN PEDIATRIC PATIENTS LESS THAN TWO YEARS OF AGE.
CAUTION SHOULD BE EXERCISED WHEN ADMINISTERING PROMETHAZINE PRODUCTS TO PEDIATRIC PATIENTS 2 YEARS OF AGE AND OLDER BECAUSE OF THE POTENTIAL FOR FATAL RESPIRATORY DEPRESSION. RESPIRATORY DEPRESSION AND APNEA, SOMETIMES ASSOCIATED WITH DEATH, ARE STRONGLY ASSOCIATED WITH PROMETHAZINE PRODUCTS AND ARE NOT DIRECTLY RELATED TO INDIVIDUALIZED WEIGHT-BASED DOSING, WHICH MIGHT OTHERWISE PERMIT SAFE ADMINISTRATION. CONCOMITANT ADMINISTRATION OF PROMETHAZINE PRODUCTS WITH OTHER RESPIRATORY DEPRESSANTS HAS AN ASSOCIATION WITH RESPIRATORY DEPRESSION, AND SOMETIMES DEATH, IN PEDIATRIC PATIENTS.
ANTIEMETICS ARE NOT RECOMMENDED FOR TREATMENT OF UNCOMPLICATED VOMITING IN PEDIATRIC PATIENTS, AND THEIR USE SHOULD BE LIMITED TO PROLONGED VOMITING OF KNOWN ETIOLOGY. THE EXTRAPYRAMIDAL SYMPTOMS WHICH CAN OCCUR SECONDARY TO PROMETHAZINE HYDROCHLORIDE ADMINISTRATION MAY BE CONFUSED WITH THE CNS SIGNS OF UNDIAGNOSED PRIMARY DISEASE, e.g., ENCEPHALOPATHY OR REYE’S SYNDROME. THE USE OF PROMETHAZINE PRODUCTS SHOULD BE AVOIDED IN PEDIATRIC PATIENTS WHOSE SIGNS AND SYMPTOMS MAY SUGGEST REYE’S SYNDROME OR OTHER HEPATIC DISEASES.
Excessively large dosages of antihistamines, including promethazine hydrochloride, in pediatric patients may cause sudden death (see OVERDOSAGE). Hallucinations and convulsions have occurred with therapeutic doses and overdoses of promethazine hydrochloride in pediatric patients. In pediatric patients who are acutely ill associated with dehydration, there is an increased susceptibility to dystonias with the use of promethazine HCl.
Other Consideration:
Administration of promethazine has been associated with reported cholestatic jaundice.
-
PRECAUTIONS
Animal reproduction studies have not been conducted with the drug combination–promethazine and dextromethorphan. It is not known whether this drug combination can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Promethazine and dextromethorphan should be given to a pregnant woman only if clearly needed.
General
Dextromethorphan should be used with caution in sedated patients, in the debilitated, and in patients confined to the supine position.
Drugs having anticholinergic properties should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy, stenosing peptic ulcer, pyloroduodenal obstruction, and bladder-neck obstruction.
Promethazine should be used cautiously in persons with cardiovascular disease or with impairment of liver function.
Information for Patients
Patients should be advised to measure Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution with an accurate measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage, especially when a half a teaspoon is measured. A pharmacist can recommend an appropriate measuring device and can provide instructions for measuring the correct dose.
Promethazine and dextromethorphan may cause marked drowsiness or impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. Ambulatory patients should be told to avoid engaging in such activities until it is known that they do not become drowsy or dizzy from promethazine and dextromethorphan therapy. Children should be supervised to avoid potential harm in bike riding or in other hazardous activities.
The concomitant use of alcohol or other central-nervous-system depressants, such as sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers, may enhance impairment (see WARNINGS – CNS Depression and PRECAUTIONS – Drug Interactions).
Patients should be advised to report any involuntary muscle movements.
Avoid prolonged exposure to the sun.
Drug Interactions
Dextromethorphan: Hyperpyrexia, hypotension, and death have been reported coincident with the coadministration of monoamine oxidase (MAO) inhibitors and products containing dextromethorphan. Thus, concomitant administration of promethazine with dextromethorphan and MAO inhibitors should be avoided (see CONTRAINDICATIONS).
Promethazine:
CNS Depressants - Promethazine may increase, prolong, or intensify the sedative action of other central-nervous-system depressants, such as alcohol, sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers; therefore, such agents should be avoided or administered in reduced dosage to patients receiving promethazine. When given concomitantly with promethazine, the dose of barbiturates should be reduced by at least one-half, and the dose of narcotics should be reduced by one-quarter to one-half. Dosage must be individualized. Excessive amounts of promethazine HCl relative to a narcotic may lead to restlessness and motor hyperactivity in the patient with pain; these symptoms usually disappear with adequate control of the pain.
Epinephrine - Because of the potential for promethazine to reverse epinephrine’s vasopressor effect, epinephrine should NOT be used to treat hypotension associated with promethazine overdose.
Anticholinergics - Concomitant use of other agents with anticholinergic properties should be undertaken with caution.
Monoamine oxidase inhibitors (MAOI) - Drug interactions, including an increased incidence of extrapyramidal effects, have been reported when some MAOI and phenothiazines are used concomitantly.
Drug/Laboratory Test Interactions
The following laboratory tests may be affected in patients who are receiving therapy with promethazine hydrochloride:
Pregnancy Tests: Diagnostic pregnancy tests based on immunological reactions between HCG and anti-HCG may result in false-negative or false-positive interpretations.
Glucose Tolerance Test: An increase in blood glucose has been reported in patients receiving promethazine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to assess the carcinogenic potential of promethazine or of dextromethorphan. There are no animal or human data concerning the carcinogenicity, mutagenicity, or impairment of fertility with these drugs. Promethazine was nonmutagenic in the Salmonella test system of Ames.
Pregnancy
Teratogenic Effects
Teratogenic effects have not been demonstrated in rat-feeding studies at doses of 6.25 and 12.5 mg/kg of promethazine HCl. These doses are from approximately 2.1 to 4.2 times the maximum recommended total daily dose of promethazine for a 50-kg subject, depending upon the indication for which the drug is prescribed. Daily doses of 25 mg/kg intraperitoneally have been found to produce fetal mortality in rats.
Specific studies to test the action of the drug on parturition, lactation, and development of the animal neonate were not done, but a general preliminary study in rats indicated no effect on these parameters. Although antihistamines have been found to produce fetal mortality in rodents, the pharmacological effects of histamine in the rodent do not parallel those in man. There are no adequate and well-controlled studies of promethazine in pregnant women.
Promethazine and dextromethorphan should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Labor and Delivery
Limited data suggest that use of promethazine HCl during labor and delivery does not have an appreciable effect on the duration of labor or delivery and does not increase the risk of need for intervention in the newborn. The effect on later growth and development of the newborn is unknown. See also “Nonteratogenic Effects”.
Nursing Mothers
It is not known whether promethazine or dextromethorphan is excreted in human milk.
Caution should be exercised when promethazine and dextromethorphan is administered to a nursing woman.
Pediatric Use
PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE ORAL SOLUTION IS CONTRAINDICATED FOR USE IN PEDIATRIC PATIENTS LESS THAN TWO YEARS OF AGE (see WARNINGS – Black Box Warning and Use in Pediatric Patients).
Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution should be used with caution in pediatric patients 2 years of age and older (seeWARNINGS – Use in Pediatric Patients).
Geriatric Use
Clinical studies of Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution and observed closely.
-
ADVERSE REACTIONS
Dextromethorphan: Dextromethorphan hydrobromide occasionally causes slight drowsiness, dizziness, and gastrointestinal disturbances.
Promethazine: Central Nervous System - Drowsiness is the most prominent CNS effect of this drug. Sedation, somnolence, blurred vision, dizziness; confusion, disorientation, and extrapyramidal symptoms such as oculogyric crisis, torticollis, and tongue protrusion; lassitude, tinnitus, incoordination, fatigue, euphoria, nervousness, diplopia, insomnia, tremors, convulsive seizures, excitation, catatonic-like states, hysteria. Hallucinations have also been reported.
Cardiovascular - Increased or decreased blood pressure, tachycardia, bradycardia, faintness.
Dermatologic - Dermatitis, photosensitivity, urticaria.
Hematologic - Leukopenia, thrombocytopenia, thrombocytopenic purpura, agranulocytosis.
Gastrointestinal - Dry mouth, nausea, vomiting, jaundice.
Respiratory - Asthma, nasal stuffiness, respiratory depression (potentially fatal) and apnea (potentially fatal) (see WARNINGS-Promethazine; Respiratory Depression).
Other - Angioneurotic edema. Neuroleptic malignant syndrome (potentially fatal) has also been reported (see WARNINGS-Promethazine; Neuroleptic Malignant Syndrome).
Paradoxical Reactions - Hyperexcitability and abnormal movements have been reported in patients following a single administration of promethazine HCl. Consideration should be given to the discontinuation of promethazine HCl and to the use of other drugs if these reactions occur. Respiratory depression, nightmares, delirium, and agitated behavior have also been reported in some of these patients.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Dextromethorphan: Dextromethorphan may produce central excitement and mental confusion. Very high doses may produce respiratory depression. One case of toxic psychosis (hyperactivity, marked visual and auditory hallucinations) after ingestion of a single dose of 20 tablets (300 mg) of dextromethorphan has been reported.
Promethazine: Signs and symptoms of overdosage with promethazine HCl range from mild depression of the central nervous system and cardiovascular system to profound hypotension, respiratory depression, unconsciousness, and sudden death. Other reported reactions include hyperreflexia, hypertonia, ataxia, athetosis, and extensor-plantar reflexes (Babinski reflex).
Stimulation may be evident, especially in children and geriatric patients. Convulsions may rarely occur. A paradoxical reaction has been reported in children receiving single doses of 75 mg to 125 mg orally, characterized by hyperexcitability and nightmares.
Atropine-like signs and symptoms–dry mouth, fixed dilated pupils, flushing, as well as gastrointestinal symptoms, may occur.
Treatment: The treatment of overdosage with promethazine and dextromethorphan is essentially symptomatic and supportive. Only in cases of extreme overdosage or individual sensitivity do vital signs including respiration, pulse, blood pressure, temperature, and EKG need to be monitored. Activated charcoal orally or by lavage may be given, or sodium or magnesium sulfate orally as a cathartic. Attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Diazepam may be used to control convulsions. Acidosis and electrolyte losses should be corrected. The antidotal efficacy of narcotic antagonists to dextromethorphan has not been established; note that any of the depressant effects of promethazine are not reversed by naloxone. Avoid analeptics, which may cause convulsions.
The treatment of choice for resulting hypotension is administration of intravenous fluids, accompanied by repositioning if indicated. In the event that vasopressors are considered for the management of severe hypotension which does not respond to intravenous fluids and repositioning, the administration of norepinephrine or phenylephrine should be considered. EPINEPHRINE SHOULD NOT BE USED, since its use in a patient with partial adrenergic blockade may further lower the blood pressure. Extrapyramidal reactions may be treated with anticholinergic antiparkinson agents, diphenhydramine, or barbiturates. Oxygen may also be administered.
Limited experience with dialysis indicates that it is not helpful.
-
DOSAGE AND ADMINISTRATION
It is important that Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution is measured with an accurate measuring device (see PRECAUTIONS-Information for Patients). A household teaspoon is not an accurate measuring device and could lead to overdosage, especially when half a teaspoon is to be measured. It is strongly recommended that an accurate measuring device be used. A pharmacist can provide an appropriate device and can provide instructions for measuring the correct dose.
Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution is contraindicated for children under 2 years of age (see WARNINGS Black Box Warning and Use in Pediatric Patients).
The average effective dose is given in the following table: Adults 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 30 mL in 24 hours. Children 6 years to under 12 years ½ to 1 teaspoonful (2.5 to 5 mL) every 4 to 6 hours, not to exceed 20 mL in 24 hours. Children 2 years to under 6 years ¼ to ½ teaspoonful (1.25 to 2.5 mL) every 4 to 6 hours, not to exceed 10 mL in 24 hours. -
HOW SUPPLIED
This preparation is a yellow to amber colored lemon-mint flavored oral solution, containing promethazine hydrochloride 6.25 mg/5 mL, dextromethorphan hydrobromide 15 mg/5 mL and alcohol 8 percent, and is available in 16 fl. oz. (473 mL) bottles, (NDC: 27808-057-01).
Keep tightly closed. Protect from light.
Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP.
Manufactured By:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852For:
Cranbury Pharmaceuticals, LLC
Monmouth Junction, NJ 08852LB8077
Rev. 02
10/2024
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 27808-057-01
Promethazine Hydrochloride and Dextromethorphan Hydrobromide Oral Solution
6.25 mg and 15 mg per 5 mL
Alcohol 8% (v/v)
Rx only
16 fl. oz. (473 mL)

-
INGREDIENTS AND APPEARANCE
PROMETHAZINE HYDROCHLORIDE AND DEXTROMETHORPHAN HYDROBROMIDE
promethazine hydrochloride and dextromethorphan hydrobromide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 27808-057 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROMETHAZINE HYDROCHLORIDE (UNII: R61ZEH7I1I) (PROMETHAZINE - UNII:FF28EJQ494) PROMETHAZINE HYDROCHLORIDE 6.25 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASCORBIC ACID (UNII: PQ6CK8PD0R) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (amber) Score Shape Size Flavor LEMON (mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 27808-057-01 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/15/2021 04/01/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091687 01/31/2014 04/01/2027 Labeler - Cranbury Pharmaceuticals, LLC (120378264)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.