HAND GEL SANITIZER- alcohol gel
Hand Gel Sanitizer by
Drug Labeling and Warnings
Hand Gel Sanitizer by is a Otc medication manufactured, distributed, or labeled by Allied Pressroom Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
This is an isopropyl alcohol based hand sanitizer manufactured following the CDC recomendation of using an alcohol concentration of more than 60 % v/v alcohol when soap and water is not available.
It is manufactured by gelling a 70 % isopropyl alcohol solution with the minimum of an acrylate polymer to create a clear gel that is easy to apply and does not evaporate as readiliy as the liquid versions to provide sufficient dwell time to be effective.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product::
- Alcohol (isopropyl alcohol) (USP or Food Chemical Codex (FCC) grade) (70%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerin (1.00 % v/v)..
- Polypropylene Glycol (1.2 % v/v)..
- Acrylate Polymer (0.3% v/v).
- Sterile distilled water or boiled cold water.
No other materials are used.
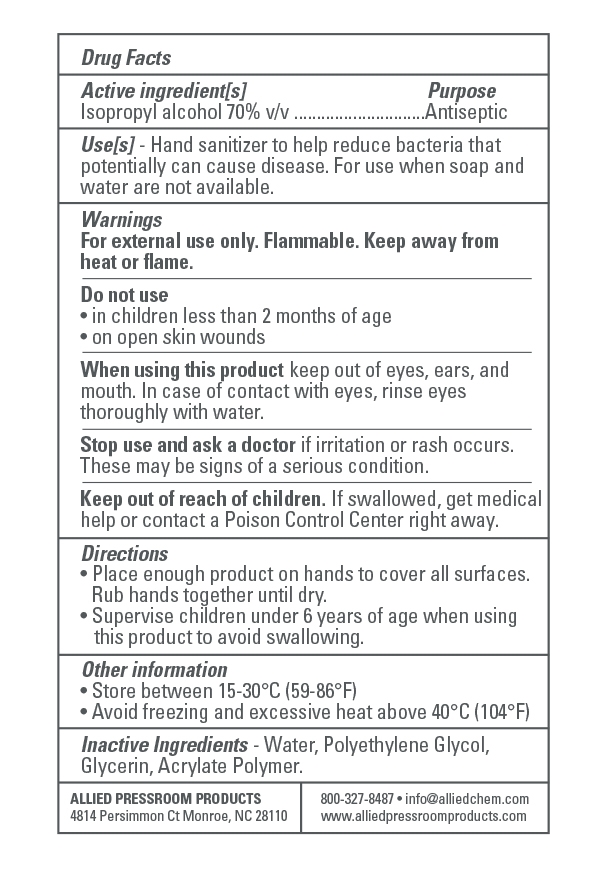
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
-
Package Label - Principal Display Panel
3785 ml NDC: 78851-444-05
 946 ml NDC: 78851-444-04
946 ml NDC: 78851-444-04
 240 ml NDC: 78851-444-03
240 ml NDC: 78851-444-03
 120 ml MDC 78851-444-02
120 ml MDC 78851-444-02
 105 ml NDC: 78851-444-01 back
105 ml NDC: 78851-444-01 back
 105 105 ml NDC: 78851-444-01 front
105 105 ml NDC: 78851-444-01 front
-
INGREDIENTS AND APPEARANCE
HAND GEL SANITIZER
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 78851-444 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) 1.2 mL in 100 mL ALLYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (LOW ALLYL METHACRYLATE) (UNII: PI80E777XB) 0.3 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 78851-444-01 105 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/23/2020 2 NDC: 78851-444-02 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/23/2020 3 NDC: 78851-444-03 240 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/23/2020 4 NDC: 78851-444-04 946 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/23/2020 5 NDC: 78851-444-05 3785 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/23/2020 Labeler - Allied Pressroom Products (069895618) Registrant - Allied Pressroom Products (069895618) Establishment Name Address ID/FEI Business Operations Allied Pressroom Products 069895618 manufacture(78851-444)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.