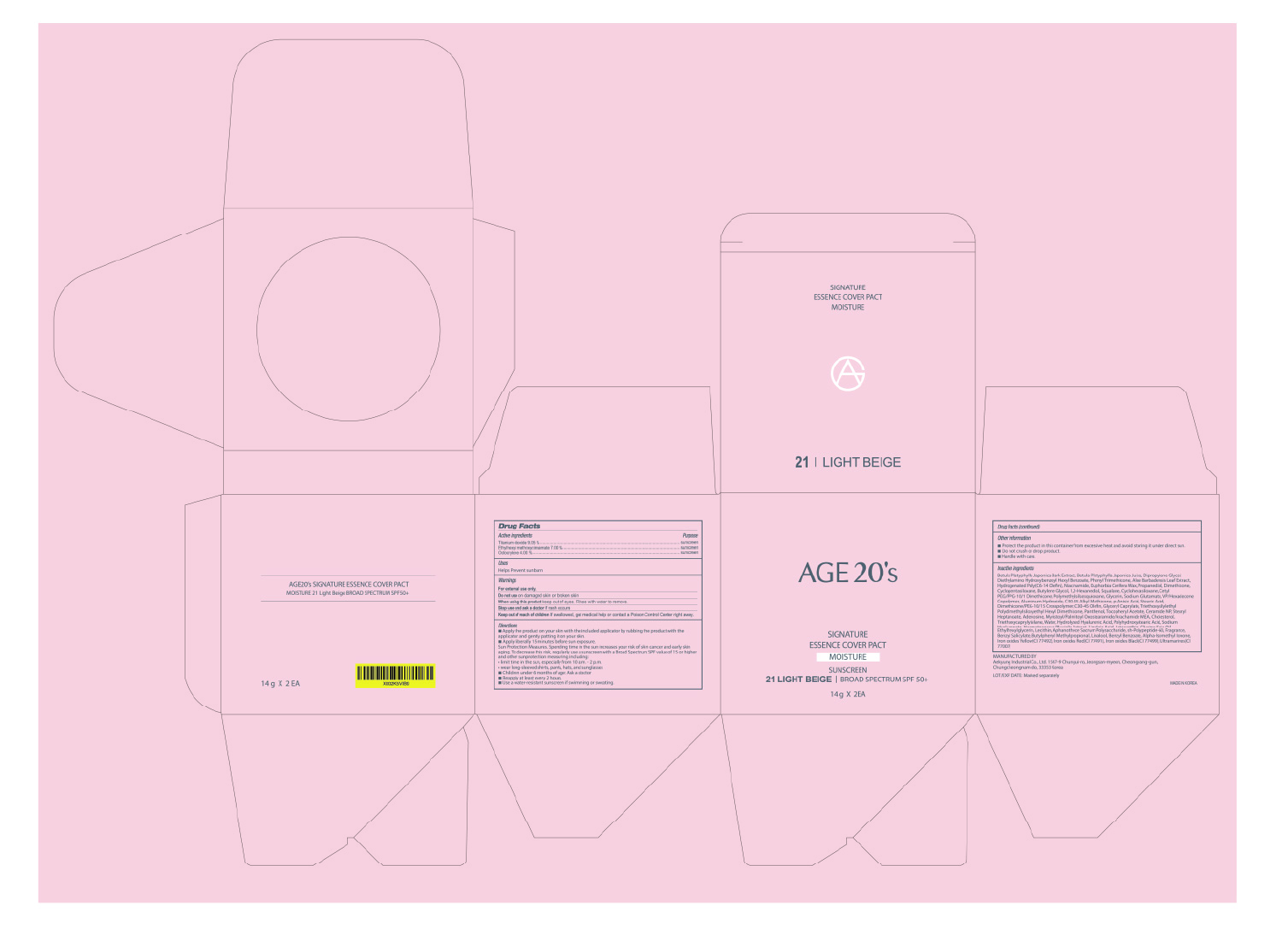
[de-listing] [67225-0624-2] AGE20'S SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE
AGE20s SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE by
Drug Labeling and Warnings
AGE20s SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE by is a Otc medication manufactured, distributed, or labeled by Aekyung Industrial Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AGE20S SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE- titanium dioxide, ethylhexyl methoxycinnamate, octocrylene cream
Aekyung Industrial Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
[de-listing] [67225-0624-2] AGE20'S SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE
Active ingredients
Titanium Dioxide 9.09 %............................ sunscreen
Ethylhexyl Methoxycinnamate 7 %............ sunscreen
Octocrylene 4 %......................................... sunscreen
Inactive ingredients
Betula Platyphylla Japonica Bark Extract, Betula Platyphylla Japonica Juice, Dipropylene Glycol,
Diethylamino Hydroxybenzoyl Hexyl Benzoate, Phenyl Trimethicone, Aloe Barbadensis Leaf Extract,
Hydrogenated Poly(C6-14 Olefin), Niacinamide, Euphorbia Cerifera (Candelilla) Wax, Propanediol,
Dimethicone, Cyclopentasiloxane, Butylene Glycol, 1,2-Hexanediol, Squalane, Cyclohexasiloxane,
Cetyl PEG/PPG-10/1 Dimethicone, Polymethylsilsesquioxane, Glycerin, Sodium Glutamate,
VP/Hexadecene Copolymer, Aluminum Hydroxide, C30-45 Alkyl Methicone, p-Anisic Acid,
Stearic Acid, Dimethicone/PEG-10/15 Crosspolymer, C30-45 Olefin, Glyceryl Caprylate,
Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Panthenol, Tocopheryl Acetate,
Ceramide NP, Stearyl Heptanoate, Adenosine, Myristoyl/Palmitoyl Oxostearamide/Arachamide MEA,
Cholesterol, Triethoxycaprylylsilane, Water, Hydrolyzed Hyaluronic Acid, Polyhydroxystearic Acid,
Sodium Hyaluronate, Haematococcus Pluvialis Extract, Linoleic Acid, Astaxanthin, Glycine Soja (Soybean) Oil,
Ethylhexylglycerin, Lecithin, Aphanothece Sacrum Polysaccharide, sh-Polypeptide-60, Fragrance, Benzyl Salicylate,
Butylphenyl Methylpropional, Linalool, Benzyl Benzoate, Alpha-Isomethyl Ionone,
Iron oxides Yellow(CI 77492), Iron oxides Red(CI 77491), Iron oxides Black(CI 77499), Ultramarines(CI 77007)
| AGE20S SIGNATURE ESSENCE COVER PACT MOISTURE 21 LIGHT BEIGE
titanium dioxide, ethylhexyl methoxycinnamate, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Aekyung Industrial Co., Ltd. (690511126) |
| Registrant - Aekyung Industrial Co., Ltd. (690511126) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aekyung Industrial Co., Ltd. | 690511126 | manufacture(67225-0624) | |