WILATE - VON WILLEBRAND FACTOR/COAGULATION FACTOR VIII COMPLEX (HUMAN) (von willebrand factor/coagulation factor viii complex- human powder, for solution
Wilate - von Willebrand Factor/Coagulation Factor VIII Complex (Human) by
Drug Labeling and Warnings
Wilate - von Willebrand Factor/Coagulation Factor VIII Complex (Human) by is a Other medication manufactured, distributed, or labeled by Octapharma USA Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Wilate safely and effectively. See full prescribing information for Wilate.
Wilate, von Willebrand Factor/Coagulation Factor VIII Complex (Human), Powder for Solution, for Intravenous Use Only.
Initial U.S. Approval: 2009INDICATIONS AND USAGE
WILATE is indicated in children and adults with von Willebrand disease for:
On-demand treatment and control of bleeding episodes
Perioperative management of bleeding
WILATE is not indicated for treatment of hemophilia A ( 1 )
DOSAGE AND ADMINISTRATION
For Intravenous Use Only
Use the following formula to determine required dosage ( 2.1 ):Required IU = body weight (BW) in kg x desired VWF:RCo rise (%) (IU/dL) x 0.5 (IU/kg per IU/dL)
Adjust dosage and duration of the substitution therapy depending on the severity of the VWD, on the location and extent of the bleeding, and on the patient’s clinical condition ( 2.1 )
Dosing recommendations ( 2.1 ):
Type of Hemorrhages/Surgery Loading Dosage (IU VWF:RCo/ kg BW) Maintenance Dosage (IU VWF:RCo/ kg BW) Therapeutic Goal Minor Hemorrhages
20-40 IU/kg
20-30 IU/kg every 12-24 hours
VWF:RCo and FVIII activity trough levels of >30%
Major Hemorrhages
40-60 IU/kg
20-40 IU/kg every 12-24 hours
VWF:RCo and FVIII activity trough levels of >50%
Minor Surgeries (including tooth extractions)
30-60 IU/kg
15-30 IU/kg or half the loading dose every 12-24 hours for up to 3 days
VWF:RCo peak level of 50% after loading dose and
trough levels of > 30% during maintenance doses
Major Surgeries
40-60 IU/kg
20-40 IU/kg or half the loading dose every 12-24 hours for up to 6 days or more
VWF:RCo peak level of 100% after loading dose and trough levels of > 50% during maintenance doses
In order to decrease the risk of perioperative thrombosis, FVIII activity levels should not exceed 250% .
DOSAGE FORMS AND STRENGTHS
WILATE is available as a sterile, lyophilized powder for reconstitution for intravenous injection, provided in the following nominal strengths per single-use vial ( 3 ):
500 IU VWF:RCo and 500 IU FVIII activities in 5 mL
1000 IU VWF:RCo and 1000 IU FVIII activities in 10 mL
CONTRAINDICATIONS
Do not use in patients with known hypersensitivity reactions, including anaphylactic or severe systemic reaction, to human plasma-derived products, any ingredient in the formulation, or components of the container ( 4 )
WARNINGS AND PRECAUTIONS
Anaphylaxis and severe hypersensitivity reactions are possible ( 5.1 ).
Thromboembolic events may occur. Monitor plasma levels of FVIII activity ( 5.2 ).
Development of neutralizing antibodies to FVIII and to VWF, especially in VWD type 3 patients, may occur ( 5.3 ).
WILATE is made from human plasma and carries the risk of transmitting infectious agents ( 5.4 ).
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) in clinical studies on VWD were hypersensitivity reactions, urticaria, and dizziness ( 6.1 )
To report SUSPECTED ADVERSE REACTIONS, contact Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised:September 2016
_____________________________________________________________________________________________________________________________________
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Thromboembolic Events
5.3 Neutralizing Antibodies
5.4 Transmissible Infectious Agents
5.5 Monitoring and Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For Intravenous Use after Reconstitution
2.1 Dose
- Initiate treatment under the supervision of a physician experienced in the treatment of coagulation disorders.
- Each vial of WILATE contains the labeled amount in International Units (IU) of von Willebrand factor (VWF) activity as measured with the Ristocetin cofactor assay (VWF:RCo), and coagulation factor VIII (FVIII) activity measured with the chromogenic substrate assay.
- The number of units of VWF:RCo and FVIII activities administered is expressed in IU, which is related to the current WHO standards for VWF and FVIII products. VWF:RCo and FVIII activities in plasma are expressed either as a percentage (relative to normal human plasma) or in IU (relative to the International Standards for VWF:RCo and FVIII activities in plasma). One IU of VWF:RCo activity is equivalent to the quantity of VWF:RCo in one mL of normal human plasma. The ratio between VWF:RCo and FVIII activities in WILATE is approximately 1:1.
- Calculate the required dosage of VWF:RCo, based on the empirical finding that 1 IU VWF:RCo per kg body weight raises the plasma VWF activity by approximately 2% of normal activity or 2 IU/dL, using the following formula:
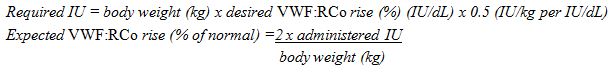
/
- Adjust the dosage and frequency of administration according to the clinical effectiveness in the individual patient.
Dosing for Hemorrhages
A guide for dosing in the treatment of major and minor hemorrhages is provided in Table 1 .
Table 1 Dosing for Treatment of Minor and Major Hemorrhages in all VWD types
Type of Hemorrhages VWF:RCo and FVIII Activity Trough Levels(% of normal) Loading Dosage(IU VWF:RCo/kg body weight) Maintenance Dosage(IU VWF:RCo/kg body weight) Frequency of Doses(hours) Duration of Therapy(days) Minor
>30
20-40
20-30
Repeat as needed
12-24
Up to 3 days
Major
>50
40-60
20-40
Repeat as needed
12-24
Up to 5-7 days
- Repeat doses as needed based upon repeat monitoring of appropriate clinical and laboratory measures.
- Perform appropriate laboratory tests on the patient’s plasma at suitable intervals to assure that adequate VWF:RCo and FVIII activity levels have been reached and are maintained.
- Because WILATE contains FVIII, continued treatment may cause an excessive rise in FVIII activity.
Dosing for Surgeries
A guide for dosing in minor and major surgeries is provided in Table 2 .
Table 2 Dosing for Treatment in Minor and Major Surgeries in all VWD Types
Type of Surgery Loading Dosage(IU VWF:RCo/kg body weight)(within 3 hours before surgery) VWF:RCo Peak Levels(% of normal) Maintenance Dosage(IU VWF:RCo/kg body weight) VWF:RCo Trough Levels(% of normal) Frequency of Doses(hours) Duration of Therapy(days) Minor
(including tooth extraction)
30-60
50
15-30
or half the loading dose
>30
12-24
Until wound healing achieved, up to 3 days
Major
40-60
100
20-40
or half the loading dose
>50
12-24
(at least 2 doses within first 24 hours after the start of surgery)
Until wound healing achieved, up to 6 days or more
In order to decrease the risk of perioperative thrombosis, FVIII activity levels should not exceed 250%.
Whereas table 2 provides a dosing range that is expected to provide the desired peak levels of VWF:RCo, the following is an example on how to calculate the loading dose based on a patient’s individual IVR which is to be determined pre-surgery.
- Prior to surgery, measure incremental in vivo recovery (IVR) and assess baseline plasma VWF:RCo activity. The IVR for VWF:RCo provides the IU/dL rise per IU/kg body weight infused and can be measured and calculated as follows:
- Measure plasma VWF:RCo at baseline
- Infuse 60 IU VWF:RCo/kg of WILATE intravenously at time 0
- Measure plasma VWF:RCo at 30 minutes after the infusion
- Calculation of the loading dose requires four values: the target peak plasma VWF:RCo level, the baseline VWF:RCo level, body weight (BW) in kilograms, and IVR. If the actual IVR calculation exceeds 2.5, use 2.5 to calculate the recommended loading dosing to avoid under-dosing. If IVR is not available, a standardized dose can be calculated based on an assumed VWF:RCo IVR of 2.0 U/dL per IU/kg of WILATE administered.
Assumption: Baseline VWF:RCo value = 10 IU/dL, and VWF:RCo target level = 100 IU/dL = Δ 90 IU/dL
Assumption: Patient’s weight = 75 kg, patient’s IVR = 1.8 (IU/dL)/(IU/kg)
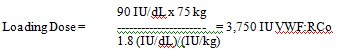
/
- When possible post surgery, perform appropriate laboratory tests on the patient’s plasma once a day after surgery to assure that adequate VWF:RCo and FVIII activity levels have been reached and are maintained. In order to decrease the risk of perioperative thrombosis, FVIII activity levels should not exceed 2 50%. [ 2 ]
2.2 Preparation and Reconstitution
- WILATE is provided with a Mix2VialTM transfer device for reconstitution of the freeze-dried powder in diluent, a 10 mL syringe, an infusion set and two alcohol swabs.
- Reconstitute the powder only directly before injection. Because WILATE contains no preservatives, use the solution immediately after reconstitution.
Instructions for Reconstitution:
/
1) Warm the concentrate (WILATE) and Diluent in the closed vials up to room temperature. If a water bath is used for warming, avoid water contact with the rubber stoppers or the caps of the vials. The temperature of the water bath should not exceed +37°C (98°F).
2) Remove the caps from the concentrate (WILATE) vial and the diluent vial and clean the rubber stoppers with an alcohol swab.
/

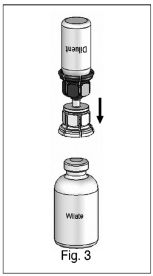
3) Peel away the lid of the outer package of the Mix2Vial™ transfer set. To maintain sterility, leave the Mix2Vial™ device in the clear outer packaging. Place the diluent vial on a level surface and hold the vial firmly. Take the Mix2Vial™ in its outer package and invert it over the diluent vial. Push the blue plastic cannula of the Mix2Vial™ firmly through the rubber stopper of the diluent vial (Fig. 1). While holding onto the diluent vial, remove the outer package from the Mix2Vial™, leaving the Mix2Vial™ attached firmly to the diluent vial (Fig. 2).
/
4) With the concentrate (WILATE) vial held firmly on a level surface, quickly invert the diluent vial with the Mix2Vial™ attached and push the transparent plastic cannula end of the Mix2Vial™ firmly through the stopper of the concentrate (WILATE) vial (Fig. 3). The diluent will be drawn into the concentrate (WILATE) vial by the vacuum. With both vials still attached, immediately start swirling the concentrate (WILATE) vial to ensure the powder is fully saturated. A slight whirlpool is formed by swirling. In order to avoid foaming, please do not shake the vial.
/
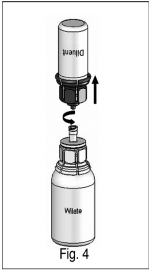
5) After 30 seconds, firmly hold both the transparent and blue parts of the Mix2Vial™. Unscrew the Mix2Vial™ into two separate pieces (Fig. 4) and discard the empty diluent vial and the blue part of the Mix2Vial™. Continue swirling until the powder in the WILATE vial has completely dissolved. This process may take several minutes.
The final solution is clear or slightly opalescent, colorless or slightly yellow. If the concentrate fails to dissolve completely or an aggregate is formed, do not use the preparation.
2.3 Administration
For intravenous injection after reconstitution only
- Inspect final solution visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not mix WILATE with other medicinal products or administer simultaneously with other intravenous preparation in the same infusion set.
- With the WILATE vial still upright, attach a plastic disposable syringe to the Mix2Vial™ (transparent plastic part). Invert the system and draw the reconstituted WILATE into the syringe.
- Once WILATE has been transferred into the syringe, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial™ from the syringe. Discard the Mix2Vial™ (transparent plastic part) and empty WILATE vial.
- Clean the intended injection site with an alcohol swab.
- Attach a suitable infusion needle to the syringe.
- Measure the patient’s pulse rate before and during the injection. If a marked increase in the pulse rate occurs, reduce the injection speed or interrupt the administration.
- Inject the solution intravenously at a slow speed of 2-4 mL/minute.
- Dispose unused product or waste material in accordance with local requirements.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
WILATE is contraindicated in patients with known hypersensitivity reactions, including anaphylactic or severe systemic reactions, to human plasma-derived products, any ingredient in the formulation [see Description ( 11 )], or components of the container.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions may occur with WILATE. Signs and symptoms include angioedema, burning and stinging at the infusion site, chills, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, and wheezing that may progress to severe anaphylaxis (including shock) with or without fever.[ 3 ] Closely monitor patients receiving WILATE and observe for any symptoms throughout the infusion period.
Because inhibitor antibodies may occur concomitantly with anaphylactic reactions, evaluate patients experiencing an anaphylactic reaction for the presence of inhibitors.[ 3 ] [See Warnings and Precautions (5. 3 )]
5.2 Thromboembolic Events
Continued treatment using a FVIII-containing VWF product may cause an excessive rise in FVIII activity[ 1 ], which may increase the risk of thromboembolic events. Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving WILATE to avoid sustained excessive VWF and FVIII activity levels.
5.3 Neutralizing Antibodies
Neutralizing antibodies (inhibitors) to FVIII and VWF in patients with VWD, especially type 3 patients, may occur. If a patient develops inhibitor to VWF (or to FVIII), the condition will manifest itself as an inadequate clinical response. Thus, if expected VWF activity plasma levels are not attained, or if bleeding is not controlled with an adequate dose or repeated dosing, perform an appropriate assay to determine whether a VWF inhibitor is present.
In patients with antibodies against VWF, VWF is not effective and WILATE administration may lead to severe adverse events. Consider other therapeutic options for such patients.
Because inhibitor antibodies may occur concomitantly with anaphylactic reactions, evaluate patients experiencing an anaphylactic reaction for the presence of inhibitors.[ 3 ] [See Warnings and Precautions (5.1)]
5.4 Transmissible Infectious Agents
WILATE is made from human plasma. Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the variant Creutzfeldt-Jakob disease (vCJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that WILATE will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, it may still potentially transmit disease. [ 5 ]
Record the batch number of the product every time WILATE is administered to a patient, and consider appropriate vaccination (against hepatitis A and B virus) of patients in regular/repeated receipt of WILATE. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Octapharma USA, Inc., at 1-866-766-4860.
5.5 Monitoring and Laboratory Tests
- Monitor plasma levels of VWF:RCo and FVIII activities in patients receiving WILATE to avoid sustained excessive VWF and FVIII activity levels, which may increase the risk of thromboembolism, particularly in patients with known clinical or laboratory risk factors.
- Monitor for development of VWF and FVIII inhibitors. Perform assays to determine whether VWF and/or FVIII inhibitor(s) is present if bleeding is not controlled with the expected dose of WILATE. [ 6 ]
-
6 ADVERSE REACTIONS
The most common adverse reactions to treatment with WILATE (≥ 1%) in patients with VWD were hypersensitivity reactions, urticaria, and dizziness. Seroconversions for antibodies to parvovirus B19 not accompanied by clinical signs of disease have been observed.
The most serious adverse reactions to treatment with WILATE in patients with VWD were hypersensitivity reactions [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed during these trials cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 157 VWD subjects (aged 1 to 83 years) received WILATE on 6769 occasions, including clinical studies that involved prophylactic use, treatment on demand, surgery, and pharmacokinetics. Of the 157 subjects, 32 (20.4%) had VWD type 1, 42 (26.8%) had VWD type 2, and 83 (52.9%) had VWD type 3; 96 (61.1%) subjects were female and 61 (38.9%) subjects were male. Overall, subjects received 11,222,241 IU of WILATE during 6355 exposure days. The most common adverse reactions were hypersensitivity reactions (3 subjects; 1.9%), urticaria and dizziness (each with 2 subjects; 1.3%). Five subjects (3.2%) showed seroconversion for antibodies to parvovirus B19 not accompanied by clinical signs of disease. Seroconversion for parvovirus B19 DNA has not been reported since implementation of minipool testing of plasma used for the manufacture of WILATE.
Immunogenicity
The immunogenicity of WILATE was specifically assessed in 97 subjects in 3 clinical studies in which subjects received 9,635,041 IU of WILATE during 5575 exposure days. No inhibitors of VWF were detected in these 3 studies. In one of 3 studies, which also assessed FVIII inhibitor development in 15 subjects, no inhibitors of FVIII were detected after administration of a total of 223,290 IU of WILATE over 419 administrations.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to WILATE with the incidence of antibodies to other products may be misleading.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the post-approval use of WILATE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to product exposure.
Post-marketing adverse reactions reported in patients treated with WILATE include, dyspnea, nausea, vomiting, rash, headache, tachycardia, flushing, hypotension, chills, cough, chest discomfort, abdominal pain, pyrexia, factor VIII inhibition, anaphylactic reaction and paresthesia.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There is no data with WILATE use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with WILATE. WILATE was given to four subjects (3 type 3 and 1 type 2B) during labor and delivery in one clinical study. Two subjects underwent vaginal delivery (type 3) and two subjects had a cesarean section (type 3/type 2B). In this study all procedures were uneventful.
In the U.S. general population, regardless of drug exposure, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk summary
There is no information regarding the presence of WILATE in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for WILATE and any potential adverse effects on the breastfed infant from WILATE or from the underlying maternal condition.
8.4 Pediatric Use
Eleven pediatric subjects with VWD between 5 to 16 years of age (8 type 3, 1 type 2, 2 type 1) were treated with WILATE for 234 bleeding episodes (BEs) in clinical studies. Treatment success was achieved in 88% of BEs [see Clinical Studies (14)]. Efficacy data during surgical prophylaxis were available for 13 pediatric VWD surgery subjects (3 subjects aged 0 to 2 years, 4 subjects aged 2 to 5 years, 3 subjects aged 6 to 12 years, and 3 subjects aged 12 to 16 years). Treatment success was achieved in 22 of 22 surgical procedures (17 minor and 5 major).
No dose adjustment is needed for pediatric patients as administered dosages were similar to those used in the adult population [see Clinical Trials ( 14 )].
-
11 DESCRIPTION
WILATE is a human plasma-derived, sterile, purified, double virus inactivated von Willebrand Factor/Coagulation Factor VIII Complex. WILATE is supplied as a lyophilized powder for reconstitution for intravenous injection. The diluent for reconstitution of the lyophilized powder is Water for Injection with 0.1% Polysorbate 80.
WILATE contains no preservative. No albumin is added as a stabilizer. WILATE is labeled with the actual VWF:RCo and FVIII activities in IU per vial. The VWF activity (VWF:RCo) is determined using a manual agglutination method referenced to the current “WHO International Standard for von Willebrand Factor Concentrate”. The FVIII activity is determined using a chromogenic substrate assay referenced to the current “WHO International Standard for Human Coagulation Factor VIII Concentrate”. The assay methodologies are according to European Pharmacopoeia (Ph.Eur.). The resulting specific activity of WILATE is ≥ 60 IU VWF:RCo and ≥ 60 IU FVIII activities per mg of total protein.
The nominal composition of WILATE is as follows:
Component Quantity/ 5 mL vial Quantity/ 10 mL vial VWF:RCo
500 IU
1000 IU
FVIII
500 IU
1000 IU
Total protein
≤ 7.5 mg
≤ 15.0 mg
Glycine
50 mg
100 mg
Sucrose
50 mg
100 mg
Sodium chloride
117 mg
234 mg
Sodium citrate
14.7 mg
29.4 mg
Calcium chloride
0.8 mg
1.5 mg
Water for injection
5 mL
10 mL
Polysorbate 80
1 mg/mL
1 mg/mL
Von Willebrand Factor/Coagulation Factor VIII complex is the active ingredient in WILATE. It is derived from large pools of human plasma collected in U.S. plasma donation centers. All plasma donations are tested for viral markers in compliance with requirements of EU CPMP and FDA guidance. In addition, the limit for the titer of human parvovirus B19 DNA in the manufacturing pool is set not to exceed 10 4 IU/mL.
The product is manufactured from cryoprecipitate, which is reconstituted in a buffer and treated with aluminum hydroxide followed by two different chromatography steps, ultra- and diafiltration, and sterile filtration. The manufacturing process includes two virus inactivation steps, namely, treatment with an organic solvent/detergent (S/D) mixture, composed of tri-n-butyl phosphate (TNBP) and Octoxynol-9, and a terminal dry heat (TDH) treatment of the lyophilized product in final container [at +100°C (212°F) for 120 minutes at a specified residual moisture level of 0.7-1.6%]. In addition, the ion-exchange chromatography step utilized during WILATE manufacturing also removes some viruses [ 8 ]. The mean cumulative virus reduction factors of these steps are summarized in Table 3 .
Table 3 Virus Reduction During WILATE Manufacturing
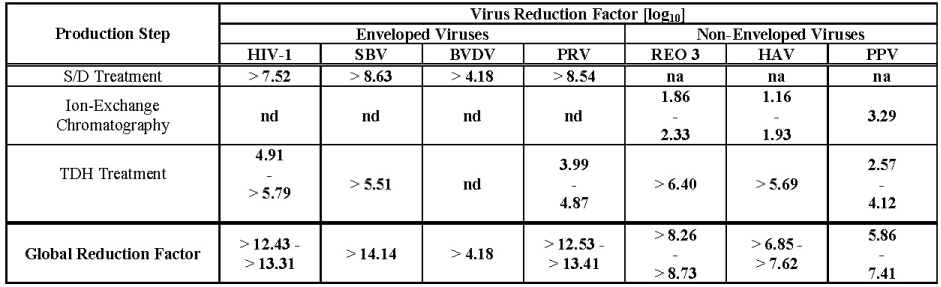
/
na: not applicable
nd: not done (S/D reagents present)
HIV-1: Human Immunodeficiency Virus - 1
SBV: Sindbis Virus
BVDV: Bovine Viral Diarrhea Virus
PRV: Pseudorabies Virus
REO 3: Reovirus Type 3
HAV: Hepatitis A Virus
PPV: Porcine Parvovirus
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
WILATE contains von Willebrand factor (VWF) and coagulation factor VIII (FVIII), constituents of normal plasma. VWF promotes platelet aggregation and platelet adhesion on damaged vascular endothelium; it also serves as a stabilizing carrier protein for the procoagulant protein FVIII, an essential cofactor in activation of factor X leading to formation of thrombin and fibrin. Patients suffering from VWD have a deficiency or abnormality of VWF. This reduction in VWF plasma concentration results in correspondingly low FVIII activity and abnormal platelet function, thereby resulting in excessive bleeding. [ 9 ] After administration, WILATE temporarily replaces missing VWF and FVIII that are needed for effective hemostasis.
12.3 Pharmacokinetics
Pharmacokinetic (PK) profiles of WILATE were determined by FVIII activity, VWF:RCo, VWF:Ag, and VWF:CB obtained from an open label, prospective, randomized, controlled, two-arm cross-over study with WILATE and a comparator product conducted at 6 sites in the US. Twenty-two subjects with inherited VWD [type 1, n=6; type 2, n=9 (6 type 2A, 1 type 2B, and 2 type 2M); and type 3, n=7] received an intravenous bolus dose of WILATE containing approximately 40 IU of VWF:RCo/kg body weight. Twenty subjects completed the study as per protocol. PK parameters for VWF:RCo and FVIII activities are summarized in Table 4 and Table 5 , respectively.
The PK parameters reported in Table 4 are based on VWF:RCo values obtained using a modified Behring Coagulation System (BCS) analytical method. The modified BCS was used because of its validated lower variability compared with the standard BCS. Measured concentrations (IU VWF:RCo/mL) are higher by the modified BCS than by the standard BCS analytical method which is used in some clinical laboratories. Dose adjusted C max and AUC determined by this modified BCS method are approximately 1.5 times higher than those by the standard BCS method. No difference has been found in incremental recovery.
Table 4 Pharmacokinetic Parameters of VWF:RCo:mean ± SD (range)
Parameters VWD type I (n = 5) VWD type II (n = 9) VWD type III (n = 6) Total (n = 20) Cmax (IU/dL)
74 ± 13
(62 - 91)
77 ± 18
(40 - 100)
79 ± 13
(65 - 102)
76 ± 15
(40 - 102)
AUC(0-inf)
(IU*hr/dL)
1633 ± 979
(984 - 3363)
1172 ± 421
(571 - 1897)
995 ± 292
(527 - 1306)
1235 ± 637
(527 - 3363)
Half-life (hrs)
24.7 ± 17.9
(11.2 - 48.5 )
15.3 ± 6.3
(6.0 - 26.4)
9.1± 2.6
(5.7 - 12.9 )
15.8 ± 11.0
(5.7 - 48.5)
CL (mL/h/kg)
3.1 ± 1.1
(1.2 - 4.1)
4.1 ± 1.7
(2.0 - 7.1)
4.2 ± 1.4
(3.0 - 6.6)
3.9 ± 1.5
(1.2 - 7.1)
Vss (mL/kg)
81.7 ± 38.5
(15.3 - 74.2)
76.6 ± 35.4
(45.3 - 158.8)
49.4 ± 16.7
(29.7 - 67.1)
69.7 ± 33.2
(29.7 - 158.8)
MRT (hrs)
32.7 ± 25.8
(15.3 - 74.2)
19.7 ± 5.6
(9.9 - 27.1)
11.9 ± 2.9
(9.2 - 15.9)
20.6 ± 14.8
(9.2 - 74.2)
Recovery
(%IU/kg)
1.8 ± 0.2
(1.5 - 2.0)
1.8 ± 0.5
(1.0 - 2.4)
2.1 ± 0.3
(1.8 - 2.6)
1.9 ± 0.4
(1.0 - 2.6)
Table 5 Pharmacokinetic Parameters of FVIII activity: mean ± SD (range) - chromogenic
Parameters VWD type I (n = 5) VWD type II (n = 8*) VWD type III (n = 6) Total (n = 19*) Cmax (IU/dL)
117.1 ± 12.1
(103 - 135)
147.2 ± 32.6
(102 - 206)
120 ± 23
(91 - 148)
112 ± 23
(59 - 148)
AUC(0-inf)
(IU*hr/dL)
1187 ± 382
(523 - 1483)
1778 ± 1430
(544 - 4821)
2670 ± 854
(1874 - 3655)
2290 ± 1045
(464 - 4424)
Half-life (hrs)
17.5 ± 4.9
(10.9 - 23.8)
23.6 ± 8.3
(12.6 - 34.7)
16.1 ± 3.1
(11.8 - 20.1)
19.6 ± 6.9
(10.9 - 34.7)
CL (mL/h/kg)
4.4 ± 3.7
(2.5 - 11.0)
2.5 ± 0.9
(1.2 - 3.5)
2.0 ± 0.6
(1.4 - 2.8)
2.9 ± 2.1
(1.2 - 11.0)
Vss (mL/kg)
95.0 ± 53.8
(57.1 - 190.0)
79.5 ± 23.1
(52.8 - 116.2)
44.2 ± 10.4
(31.8 - 57.1)
72.4 ± 36.2
(31.8 - 190.0)
MRT (hrs)
24.1 ± 5.5(17.2 - 31.5)
35.1 ± 14.2
(17.5 - 61.6)
23.0 ± 3.7
(18.0 - 27.7)
28.4 ± 11.1
(17.2 - 61.6)
Recovery
(%IU/kg)
1.9 ± 0.5
(1.1 - 2.5)
2.2 ± 0.4
(1.6 - 2.8)
2.5 ± 0.5
(2.0 - 3.0)
2.2 ± 0.5
(1.1 - 3.0)
C max = peak concentration; AUC = area under curve; CL = clearance; Vss = volume of distribution at steady state; MRT = mean residence time
-
14 CLINICAL STUDIES
Treatment of Bleeding Episodes
Clinical efficacy of WILATE in the control of bleeding in subjects with VWD was determined in four prospective, open-label, non-controlled clinical studies (excluding PK study). For inclusion in the studies, subjects had to have inherited VWD (any type) that did not respond to desmopressin acetate. Subjects aged ≥12 to ≤65 years were eligible to enter three of the four studies, and subjects aged ≥6 to ≤85 years were eligible for one study. Exclusion criteria included the administration of other plasma-derived or blood products or desmopressin acetate 15 days before study entry, administration of acetylsalicylic acid 7 days before study entry, symptomatic infection, past or present inhibitor activity (3 studies), and severe liver or kidney disease (3 studies). A total of 70 VWD subjects with a mean age of 37 years (range 5–77 years) were enrolled in the studies, of whom 37 were type 3 and 30 were male. The total number of exposure days to WILATE for all investigations across the four studies ranged from 202 to 4917 days. Treated BEs were analyzed for efficacy using a set of objective criteria in addition to a subjective 4-point hemostatic efficacy scale (excellent, good, moderate and none) which was determined at the discretion of the investigator. In assessing efficacy using the objective criteria, treatment of a bleeding episode was classified as a success when none of the criteria listed below were met:
- the episode was additionally treated with another VWF-containing product (excluding whole blood)
- the subject received a blood transfusion during the episode
- follow-up treatment with a daily dosage of WILATE that was greater than or equal to 50% (≥ 50%) above the initial dose (for bleeding episodes with more than 1 day of treatment)
- treatment duration of more than 4 days (> 4 days) in cases of severe bleeding (other than gastrointestinal)
- treatment duration of more than 3 days (> 3 days) in cases of moderate bleeding (other than gastrointestinal)
- treatment duration of more than 2 days (> 2 days) in cases of minor bleeding (other than gastrointestinal)
- the last efficacy rating of the bleeding episode was 'moderate' or 'none'
BEs treated with WILATE are summarized for all subjects (n=45) and subjects aged 5–16 years (n=11) in Table 6 . Among the 70 VWD subjects administered WILATE in clinical studies (excluding the PK study), 45 received on demand treatment for 1068 BEs. Using the above objective criteria, corresponding efficacy for each bleeding event was rated as being successful in 84% of the episodes. In these 45 subjects with BEs, 93% of the successfully treated BEs occurred in VWD type 3 subjects (n=25). In 11 pediatric patients aged 5–16 years, efficacy was rated as being successful in 87.6% of BEs.
Table 6 Proportion of Successful Treatments of Bleeding Episodes with WILATE
Number of BEs* Number of successfully treated BEs % Successes (95% CI) All subjects (n=45)
1068
898
84.1 (81.8-86.2
Subjects 5–16 years (n=11)
234
205
87.6 (82.7-91.5)
Dosing information for 972 successfully treated “bleeding episodes” (1423 infusions), and 211 successfully treated bleeding episodes (289 infusions) in subjects aged 5–16 years, for regional bleeding is summarized in Table 7 . For the purpose of assigning success/failure to regional bleeding that occurred at the same time, bleeding at different sites over the same time span was counted as separate BEs. Thus, the number of these “episodes” is different from that in the overall evaluation for success/failure of WILATE in the treatment of BEs in Table 6 . The majority of BEs were treated for 1-3 days. In subjects with GI bleeds, the duration for product use to control bleeding was longer (up to 7 days).
Table 7 Administered Dosages (VWF:RCo in IU/kg) in Bleeding Episodes* Successfully Treated with WILATE: Mean ± SD (Range)
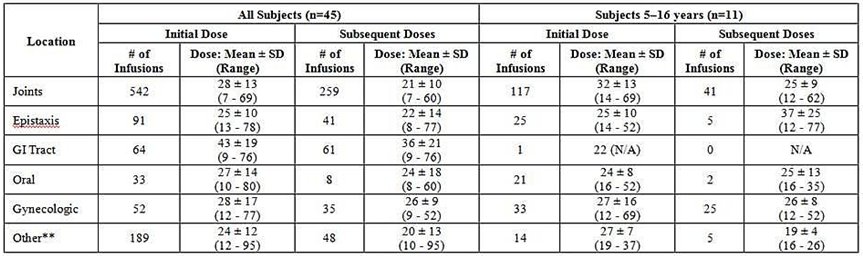
/
*For the purpose of this analysis, bleeding at each site is counted as a separate “episode”.
**“Other” includes mostly muscle bleeds, hematuria, ecchymosis, hematoma and other miscellaneous sites of bleeding.
Prevention of Bleeding in Surgery (Perioperative Management)
A prospective, open-label, single-arm, uncontrolled, multi-center clinical study was conducted to investigate the safety and hemostatic efficacy of WILATE in 28 subjects (19 female and 9 male) who underwent 30 surgeries. Two female subjects underwent 2 surgeries each. Subjects ranged in age from 12 to 74 years (median = 36). Three subjects were between 12 and 17 years old and 4 were 65 years or older. Six subjects had type 1 VWD, 1 had type 2A, 1 type 2B, and 20 had type 3 VWD. One type 1 and one type 3 subject had 2 surgeries each.
Twenty-one surgeries were classified as major (e.g. orthopedic joint replacement, cesarean section and vaginal deliveries, laminectomy, tonsillectomy, appendectomy, 3 rd molar extractions) and 9 were classified as minor (e.g. menisectomy, teeth extractions other than 3 rd molars, septoplasty, biopsy). Seven surgeries (3 major, 4 minor) were performed in type 1 VWD subjects, 2 surgeries (1 major, 1 minor) were performed in type 2 (A/B) VWD subjects, and 21 surgeries (17 major, 4 minor) were performed in type 3 VWD subjects. The types of surgery for the 9 minor procedures were: dental (n=5, 55.6%); orthopedic (n=2, 22.2%); ophthalmologic (n=1, 11.1%); and ear, nose and throat (n=1, 11.1%). The types of surgery for the 21 major procedures were: orthopedic (n=8, 38.1%); obstetric/gynecological (n=5, 23.8%); gastrointestinal (n=4, 19.0%); dental (n=2, 9.5%); and ear, nose and throat (n=2, 9.5%). The type of surgery according to VWD type were: VWD type 1 (n=7) – dental (n=4, 57.1%), orthopedic (n=2, 28.6%), and ear, nose and throat (n=1, 14.3%); VWD type 2 (A/B) (n=2) – orthopedic (n=1, 50%), and obstetric/gynecological (n=1, 50%); VWD type 3 (n=21) – orthopedic (n=7, 33.3%), gastrointestinal (n=4, 19.0%), obstetric/gynecological (n=4, 19.0%), dental (n=3, 14.3%), ear, nose and throat (n=2, 9.5%), and ophthalmologic (n=1, 4.8%).
Dosing was individualized based on in-vivo recovery results performed before surgery. Mean total loading dose per infusion was 51.4 IU/kg (median 52.1 IU/kg; range 27-77 IU/kg). Major surgeries required a mean loading dose of 54.7 IU/kg (median 55.5 IU/kg; range 36-69 IU/kg) in comparison with a mean loading dose of 41.9 IU/kg (median 37.5 IU/kg; range 27−77 IU/kg) for minor surgeries. Mean total maintenance dose per infusion was 28.5 IU/kg (median 28.5 IU/kg; range 8-63 IU/kg). Major surgeries required a mean maintenance infusion of 29.6 IU/kg (median 30 IU/kg; range 8-63 IU/kg) in comparison with a mean maintenance infusion of 21.6 IU/kg (median 20.6 IU/kg; range 14-38 IU/kg) for minor surgeries.
Efficacy of WILATE in surgical procedures was assessed by the surgeon at the conclusion of surgery and by the investigator-hematologist at 24 hours following completion of the final maintenance dose. Efficacy of WILATE was assessed using a stringent and objective 4-point ordinal efficacy scale (excellent, good, moderate, or none) based on estimated expected versus actual blood loss, transfusion requirements and post-operative bleeding and oozing. A rating of excellent or good was required to declare the outcome a success. An independent data monitoring committee (IDMC) additionally conducted an independent post hoc adjudication of intra- and post-operative assessments made by the surgeon/investigator-hematologist. In situations where the IDMC’s assessment differed from that of the surgeon and/or investigator-hematologist, the IDMC assessment took priority.
The overall efficacy of WILATE treatment for surgical procedures in this study was 96.7%. Treatment with WILATE was successful in all minor surgeries and in 95.2% of major surgeries ( Table 13 ). It was also successful in all surgical procedures in VWD type 3 and type 2 subjects and in 85.7% of procedures in VWD type 1 subjects ( Table 14 ). One failure was reported in a VWD type 1 subject undergoing lumbar laminectomy (major surgery) who experienced slightly greater blood loss (25 mL) than the expected maximum (20 mL).
Table 13 Hemostatic Efficacy Assessment by Severity of Surgery as Adjudicated by the IDMC (n=30)
Minor (n=9) Major (n=21) All surgeries (n=30) Efficacy grade
n (%)
Rate
98.75% CI
n (%)
Rate
98.75% CI
n (%)
Rate
98.75% CI
Success
9 (100)
1.000
0.569, 1.000
20 (95.2)
0.952
0.704, 1.000
29 (96.7)
0.967
0.784, 1.000
Failure
0
1 (4.8)
1 (3.3)
Table 14 Hemostatic Efficacy Assessment by Type of VWD as Adjudicated by the IDMC (n=30)
VWD type 1 VWD type 2 VWD type 3 Efficacy grade
n (%)
Rate
98.75% CI
n (%)
Rate
98.75% CI
n (%)
Rate
98.75% CI
Overall IDMC assessment
Success
6 (85.7)
0.857
0.328, 0.999
2 (100)
1.000
0.079, 1.000
21 (100)
1.000
0.785, 1.000
Failure
1 (14.3)
0
0
In this study, actual blood loss (median) was lower than the expected maximal estimated blood loss in all types of surgeries. The actual blood loss was also lower than the average predicted blood loss in minor surgeries and equal in major surgeries ( Table 15 ).
Table 15 Expected and Actual Estimated Blood Loss during Surgery
Estimated Blood Loss Minor Surgery (n = 9) Major Surgery (n= 21) Expected Maximum - Median (range) mL
50 (1-200)
500 (20-2000)
Expected Average – Median (range) mL
20 (1-100)
100 (5-1500)
Actual - Median (range) mL
15 (1-50)
100 (0-1200)
Three subjects received transfusions following surgery due to anemia and low hemoglobin values seen post-operatively.
-
15 REFERENCES
- Mannucci P.M.: Venous thromboembolism in von Willebrand disease. Thromb Haemost 2002;88:378-379
- Nichols WL, Hultin MB, et al. von Willebrand Disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia 2008; 14: 171-232
- Mollison.P.L., Engelfriet C.P., Contreras M.: Some unfavourable effects of transfusion; in Klein H.G., Anstee D.J. (eds): Mollison's Blood Transfusion in Clinical Medicine. Blackwell Publishing, 2005, pp 666-700
- Mannucci P.M., Federici A.B.: Antibodies to von Willebrand factor in von Willebrand disease; in Aledort L.M. (ed): Inhibitors to Coagulation Factors. New York, Plenum Press, 1995, pp 87-92
- Kasper, C.K., Kipnis, S.A. Hepatitis and Clotting Factor Concentrates. JAMA1972; 221:510
- Biggs, R. Jaundice and Antibodies Directed Against Factors VIII and IX in Patients Treated for Haemophilia or Christmas Disease in the United Kingdom. Br J Haematol 1974; 26:313-329
- Azzi A., Morfini M., Mannucci P.M.: The transfusion-associated transmission of parvovirus B19. Transfus.Med.Rev. 1999;13:194-204
- Stadler M., et al. Characterisation of a novel high-purity, double virus inactivated von Willebrand Factor and Factor VIII concentrate (Wilate). Biologicals 2006; 34:281-288
- Mannucci P.M.: Treatment of von Willebrand's disease. New England Journal of Medicine 2004;351:683-694
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- WILATE is supplied in packages comprising of a single-dose vial of powder and a vial of diluent (Water for Injection with 0.1% Polysorbate 80), together with a Mix2VialTM transfer device, a 10-mL syringe, an infusion set and two alcohol swabs.
Kit NDC Number Size Protein Amount 68982-182-01
68982-182-02
500 IU VWF:RCo and 500 IU FVIII activities in 5 mL
1000 IU VWF:RCo and 1000 IU FVIII activities in 10 mL
≤ 7.5 mg
≤ 15.0 mg
- Each vial of WILATE contains the labeled amount of IU of VWF:RCo activity as measured using a manual agglutination method, and IU of FVIII activity measured with a chromogenic substrate assay.
- Components used in the packaging of WILATE are not made with natural rubber latex.
- Store WILATE for up to 36 months at +2°C to +8°C (36°F to 46°F) in the original containers to protect from light from the date of manufacture. Within this period, WILATE may be stored for a period of up to 6 months at room temperature (maximum of +25°C or 77°F). The starting date of room temperature storage should be clearly recorded on the product carton. Once stored at room temperature, the product must not be returned to the refrigerator. The shelf-life then expires after the storage at room temperature, or the expiration date on the product vial, whichever is earliest. Do not freeze.
- Do not use after the expiration date.
- Reconstitute the WILATE powder only directly before injection. Use the solution immediately after reconstitution and discard any remaining solution.
-
17 PATIENT COUNSELING INFORMATION
- Advise the patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If allergic symptoms occur, advise patients to discontinue the administration immediately and contact their physician to administer appropriate emergency treatment [see Warnings and Precautions ( 5.1 )].
- Inform patients that undergoing multiple treatments with WILATE may increase the risk of thrombotic events thereby requiring frequent monitoring of plasma VWF:RCo and FVIII activities. [see Warnings and Precautions ( 5.2 )].
- Inform patients that there is a potential of developing inhibitors to VWF, leading to an inadequate clinical response. Thus, if the expected VWF activity plasma levels are not attained, or if bleeding is not controlled with an adequate dose or repeated dosing, contact the treating physician.[ 3 ] [see Warnings and Precautions (5. 3 )].
- Inform patients that despite procedures for screening donors and plasma as well as those for inactivation or removal of infectious agents, the possibility of transmitting infective agents with plasma-derived products cannot be totally excluded [see Warnings and Precautions ( 5.4 )].
Manufactured by:
Octapharma Pharmazeutika Produktionsges.m.b.H.
Oberlaaer Strasse 235
A-1100 Vienna, Austria
U.S. License No. 1646
Distributed by:
Octapharma USA Inc.
121 River Street, 12th floor
Hoboken, NJ 07030
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
Patient Information
WILATE
von Willebrand Factor/Coagulation Factor VIII Complex (Human)
Please read this Patient Information carefully before using WILATE and each time you get a refill, as there may be new information. This Patient Information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is WILATE ?
WILATE is an injectable medicine that is used to treat bleeding in adults and children with severe von Willebrand disease (VWD) as well as patients with mild or moderate VWD in whom the use of desmopressin is known or suspected to be ineffective or contraindicated.
WILATE is also used for the prevention of excessive bleeding during and after surgery in adults and children with VWD.
Who should not use WILATE ?
You should not use WILATE if you are allergic to von Willebrand Factor or Factor VIII or any of the other ingredients of WILATE.
What should I tell my healthcare provider before using WILATE ?
Talk to your healthcare provider about any medical conditions that you have or have had.
Tell your healthcare provider about all of the prescription and non-prescription medicines you take, including over-the-counter medicines, dietary supplements, or herbal medicines.
Tell your healthcare provider if you are pregnant or nursing because WILATE might not be right for you.
How should I use WILATE ?
You get WILATE as an infusion into your vein after reconstitution of the lyophilized powder by mixing it with the supplied 5 or 10 milliliter sterile solvent (Water for Injection with 0.1% Polysorbate) with the supplied transfer device (see instruction for reconstitution and injection of WILATE).
Your healthcare provider will instruct you on how to do reconstitutions and infusions using sterile technique on your own or with the help of a family member, and they may watch you give yourself the first dose of WILATE.
You must carefully follow your healthcare provider’s instructions regarding the dose and schedule for infusing WILATE so that your treatment will work the best that it can for you.
WILATE comes in different dosage strengths. The actual number of international units (IU) of von Willebrand Factor and Factor VIII in the vial will be imprinted on the label and box. Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
Contact your healthcare provider right away if bleeding is not controlled after using WILATE.
Talk to your healthcare provider before travelling. Plan to bring enough WILATE for your treatment during this time.
If you forget to use WILATE, do not inject a double dose to make up for the forgotten dose. Proceed with the next infusion as scheduled.
Do not stop using WILATE without consulting with your healthcare provider.
What are the possible side-effects of WILATE ?
Allergic reactions may occur. Call your healthcare provider or emergency department right away if you have any of the following symptoms: difficulty breathing, chest tightness, swelling of the face, rash or hives.
Because it is made from human blood, there is the risk that WILATE can potentially transmit disease or cause allergic reactions. Tell your healthcare provider if you have low-grade fever, rash, joint pain, nausea, vomiting, feeling tired, and yellowing of the skin.
Common side effects of WILATE are hypersensitivity reactions including urticaria (rash) dizziness, and testing positive for a common virus (parvovirus B19).
These are not all of the possible side effects from WILATE. Ask your healthcare provider for more information. You are encouraged to report side effects to Octapharma USA Inc. at 1-866-766-4860 or FDA at 1-800-FDA-1088.
Talk to your healthcare provider about any side-effect that bothers you or that does not go away.
How should I store WILATE ?
Keep WILATE in its original box to protect it from exposure to light. Do not freeze WILATE .
You can store WILATE for up to 36 months at +2°C to +8°C (36°F to 46°F) from date of manufacture. Within this period, WILATE may be stored for a period of up to 6 months at room temperature (maximum of +25°C or 77°F). Note on the carton the date which the product was removed from the refrigerator.
After storage at room temperature, the product must be used or discarded, and it must not be put back into the refrigerator.
Do not use WILATE after the expiration date printed on the vial.
Do not use WILATE if the reconstituted solution is cloudy, contains particles, or is not colorless.
Use WILATE immediately after mixing.
Dispose all materials, including any unused WILATE , in an appropriate container.
What else should I know about WILATE ?
Do not use WILATE for a medical condition for which it was not prescribed. Do not share WILATE with other people, even if they have the same diagnosis and symptoms that you have.
Resources at Octapharma available to patients
For more product information on WILATE, please visit www.wilateusa.com
For more information on patient assistance programs that are available to you, please contact the Octapharma Patient Support Center at 1-800-554-4440.
Manufactured by
Octapharma Pharmazeutika Produktionsges.m.b.H., Oberlaaer Strasse 235, A-1100 Vienna, Austria
U.S. License No. 1646
Distributed by
Octapharma USA, Inc., 121 River Street, 12 th floor, Hoboken, NJ 07030
wilate® is a registered trademark of Octapharma.
-
INSTRUCTIONS FOR USE
WILATE
von Willebrand Factor/Coagulation Factor VIII Complex (Human)
WILATE is supplied as a powder. Before it can be infused in your vein (intravenous injection), you must mix the powder with the supplied liquid diluent.
Do not attempt to do an infusion to yourself unless you have been taught how by your healthcare provider or hemophilia center.
Always follow the specific instructions given by your healthcare provider. The steps listed below are general guidelines for using WILATE. If you are unsure of the procedures, please call you healthcare provider before using WILATE. Your healthcare provider will prescribe the dose that you should take.
Preparation and Reconstitution
- WILATE is provided with a Mix2VialTM transfer device for reconstitution of the WILATE powder in diluent.
- Reconstitute the powder only directly before injection.
- Because WILATE contains no preservatives, use the solution immediately after reconstitution.
/
1) Warm the WILATE and diluent vials in the closed vials up to room temperature. If a water bath is used for warming, avoid water contact with the rubber stoppers or the caps of the vials. The temperature of the water bath should not exceed +37°C (98°F).
2) Remove the caps from the WILATE vial and the diluent vial and clean the rubber stoppers with an alcohol swab.
/

3) Peel away the lid of the outer package of the Mix2Vial™ transfer set. To maintain sterility, leave the Mix2Vial™ device in the clear outer packaging. Place the diluent vial on a level surface and hold the vial firmly. Take the Mix2Vial™ in its outer package and invert it over the diluent vial. Push the blue plastic cannula of the Mix2Vial™ firmly through the rubber stopper of the diluent vial (Fig. 1). While holding onto the diluent vial, remove the outer package from the Mix2Vial™, leaving the Mix2Vial™ attached firmly to the diluent vial (Fig. 2).
/
4) With the WILATE vial held firmly on a level surface, quickly invert the diluent vial with the Mix2Vial™ attached and push the transparent plastic cannula end of the Mix2Vial™ firmly through the stopper of the WILATE vial (Fig. 3). The diluent will be drawn into the WILATE vial by the vacuum. With both vials still attached, immediately start swirling the concentrate (WILATE) vial to ensure the powder is fully saturated. A slight whirlpool is formed by swirling. In order to avoid foaming, please do not shake the vial.
/
5) After 30 second, firmly hold both the transparent and blue parts of the Mix2Vial™. Unscrew the Mix2Vial™ into two separate pieces(Fig. 4) and discard the empty diluent vial and the blue part of the Mix2Vial™. Continue swirling until the powder in the WILATE vial has completely dissolved. This process may take several minutes.
The final solution is clear or slightly opalescent, colorless or slightly yellow. If the concentrate fails to dissolve completely or an aggregate is formed, do not use the preparation.
Administration
For intravenous use after reconstitution only.
- Inspect final solution visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not mix WILATE with other medicinal products or administer simultaneously with other intravenous preparation in the same infusion set.
- With the WILATE vial still upright, attach a plastic disposable syringe to the Mix2Vial™ (transparent plastic part). Invert the system and draw the reconstituted WILATE into the syringe.
- Once WILATE has been transferred into the syringe, firmly hold the barrel of the syringe (keeping the syringe plunger facing down) and detach the Mix2Vial™ from the syringe. Discard the Mix2Vial™ (transparent plastic part) and empty WILATE vial.
- Clean the intended injection site with an alcohol swab.
- Attach a suitable infusion needle to the syringe.
- Measure the patient’s pulse rate before and during the injection. If a marked increase in the pulse rate occurs, reduce the injection speed or interrupt the administration.
- Inject the solution intravenously at a slow speed of 2-4 mL/minute.
- Dispose unused product or waste material in accordance with local requirements.
For more product information on WILATE, please visit www.wilateusa.com
Manufactured by
Octapharma Pharmazeutika Produktionsges.m.b.H., Oberlaaer Strasse 235, A-1100 Vienna, Austria
U.S. License No. 1646
Distributed by
Octapharma USA, Inc., 121 River Street, 12 th floor, Hoboken, NJ 07030
wilate® is a registered trademark of Octapharma.
Issued September 2016.
-
PRINCIPAL DISPLAY PANEL
von Willebrand Factor/Coagulation Factor VIII Complex (Human)
Octapharma Pharmazeutika Produktionsges.m.b.H
500IU/5mL
NDC: 68982-182-01

/
1000IU/10mL
NDC: 68982-182-02

/
-
INGREDIENTS AND APPEARANCE
WILATE - VON WILLEBRAND FACTOR/COAGULATION FACTOR VIII COMPLEX (HUMAN)
von willebrand factor/coagulation factor viii complex (human) powder, for solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68982-182 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIHEMOPHILIC FACTOR HUMAN (UNII: 839MOZ74GK) (ANTIHEMOPHILIC FACTOR HUMAN - UNII:839MOZ74GK) ANTIHEMOPHILIC FACTOR HUMAN 100 [iU] in 1 mL VON WILLEBRAND FACTOR HUMAN (UNII: ZE22NE22F1) (VON WILLEBRAND FACTOR HUMAN - UNII:ZE22NE22F1) VON WILLEBRAND FACTOR HUMAN 100 [iU] in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68982-182-02 10 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC: 68982-182-01 5 mL in 1 VIAL; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125251 12/04/2009 Labeler - Octapharma USA Inc (606121163)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.