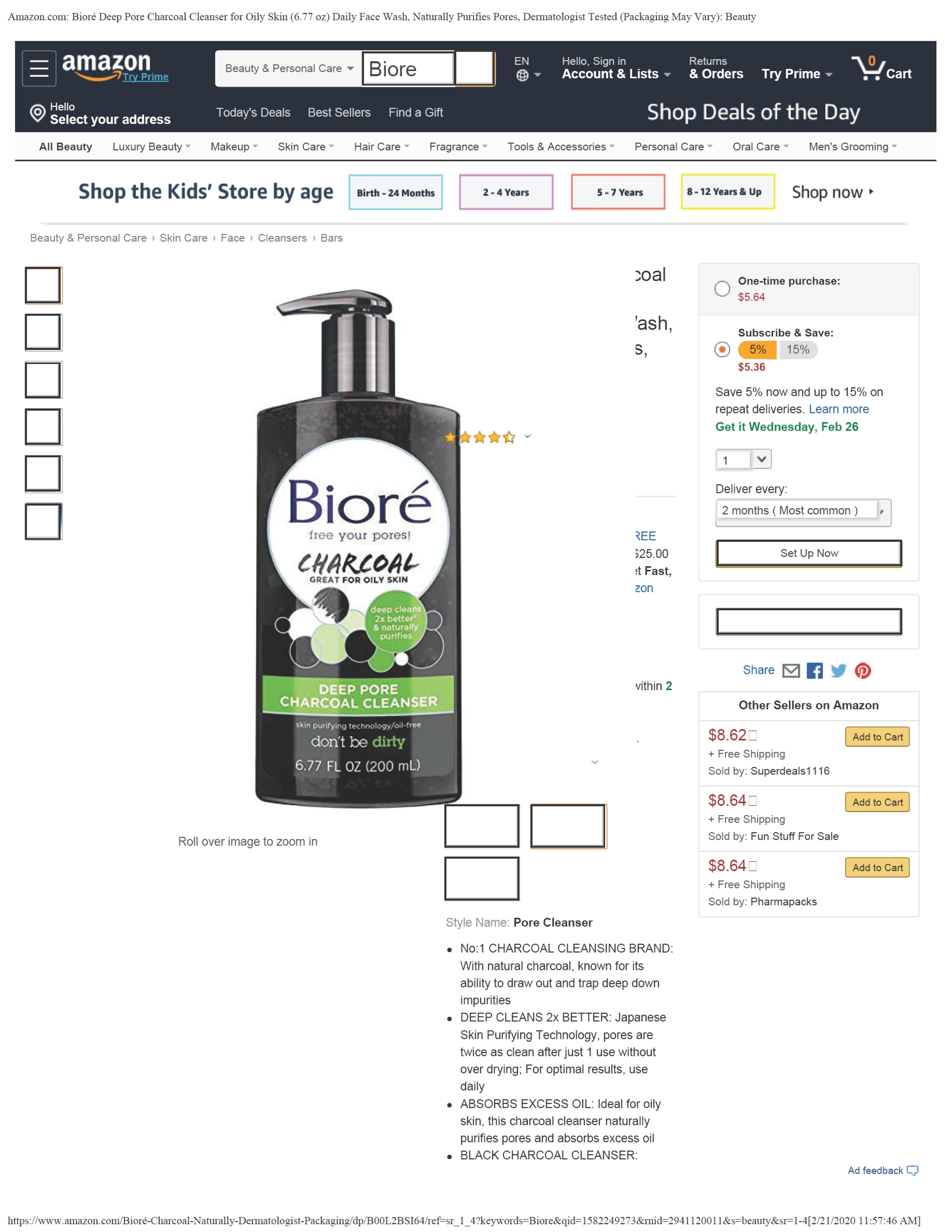
Biore by Kao USA Inc. Biore Daily Detox Toner
Biore by
Drug Labeling and Warnings
Biore by is a Otc medication manufactured, distributed, or labeled by Kao USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIORE DAILY DETOX ACNE TONER- salicylic acid liquid
Kao USA Inc.
----------
Biore Daily Detox Toner
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Cleanse skin thoroughly before applying product
- Cover the entire affected area with a thin layer 1 to 3 times daily using a cotton ball or pad.
- Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredientsWater, Alcohol Denat., Isopropyl Alcohol, PEG-7 Glyceryl Cocoate, Hamamelis Virginiana (Witch Hazel) Water, Glycerin, Polysorbate 20, Sodium hydroxide, Sodium Citrate, Alcohol, Citric Acid, Menthol, Benzophenonone-4, Disodium EDTA, Fragrance, Cannabis Sativa (Hemp) Seed Oil, Propylene Glycol, Camelia Sinensis Leaf Extract.
| BIORE
DAILY DETOX ACNE TONER
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Kao USA Inc. (004251617) |
Revised: 12/2025
<
Document Id: 4744cb8a-7afa-f8e4-e063-6294a90ab924
Set id: a8f1af64-3ffc-7733-e053-2a95a90a046f
Version: 2
Effective Time: 20251231
Trademark Results [Biore]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIORE 98041507 not registered Live/Pending |
Wada Ryuichi 2023-06-14 |
 BIORE 79211943 5403993 Live/Registered |
Remei AG 2017-05-05 |
 BIORE 79180954 5061577 Live/Registered |
Biore-Stiftung 2015-10-31 |
 BIORE 79023479 3252012 Dead/Cancelled |
Remei AG 2006-04-28 |
 BIORE 79002630 3145650 Dead/Cancelled |
Remei AG 2004-04-07 |
 BIORE 79002437 3035908 Dead/Cancelled |
Biore-Stiftung 2004-04-07 |
 BIORE 75263125 2194293 Live/Registered |
Kao Kabushiki Kaisha 1997-03-25 |
 BIORE 73789224 1620721 Live/Registered |
KAO KABUSHIKI KAISHA 1989-03-27 |
 BIORE 73418486 not registered Dead/Abandoned |
KAO SEKKEN KABUSHIKI KAISHA 1983-03-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
