Bismuth Subsalicylate 262 mg
Bismuth Subsalicylate 262 mg by
Drug Labeling and Warnings
Bismuth Subsalicylate 262 mg by is a Otc medication manufactured, distributed, or labeled by Preferred Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
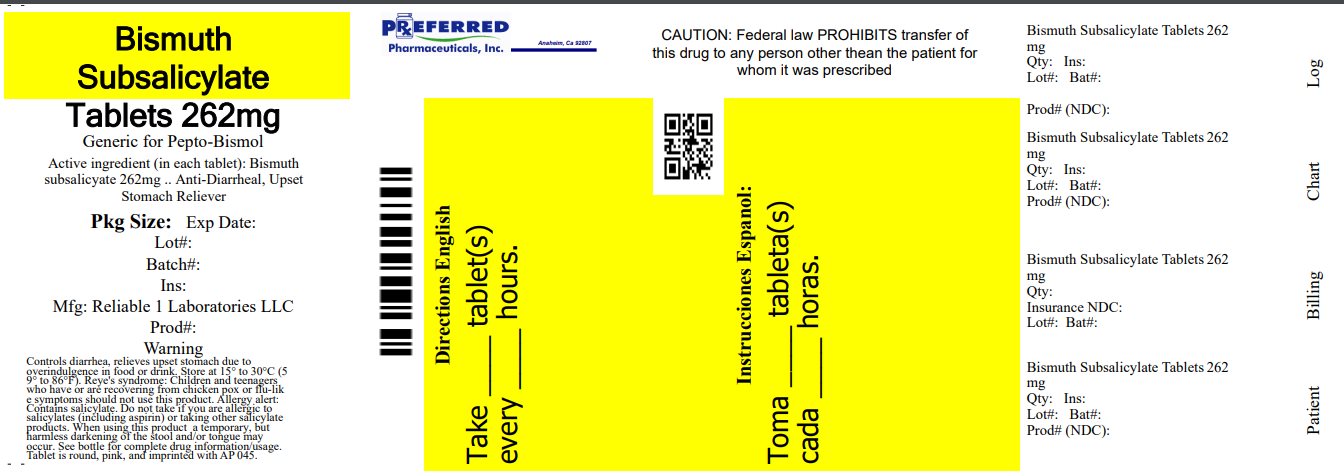
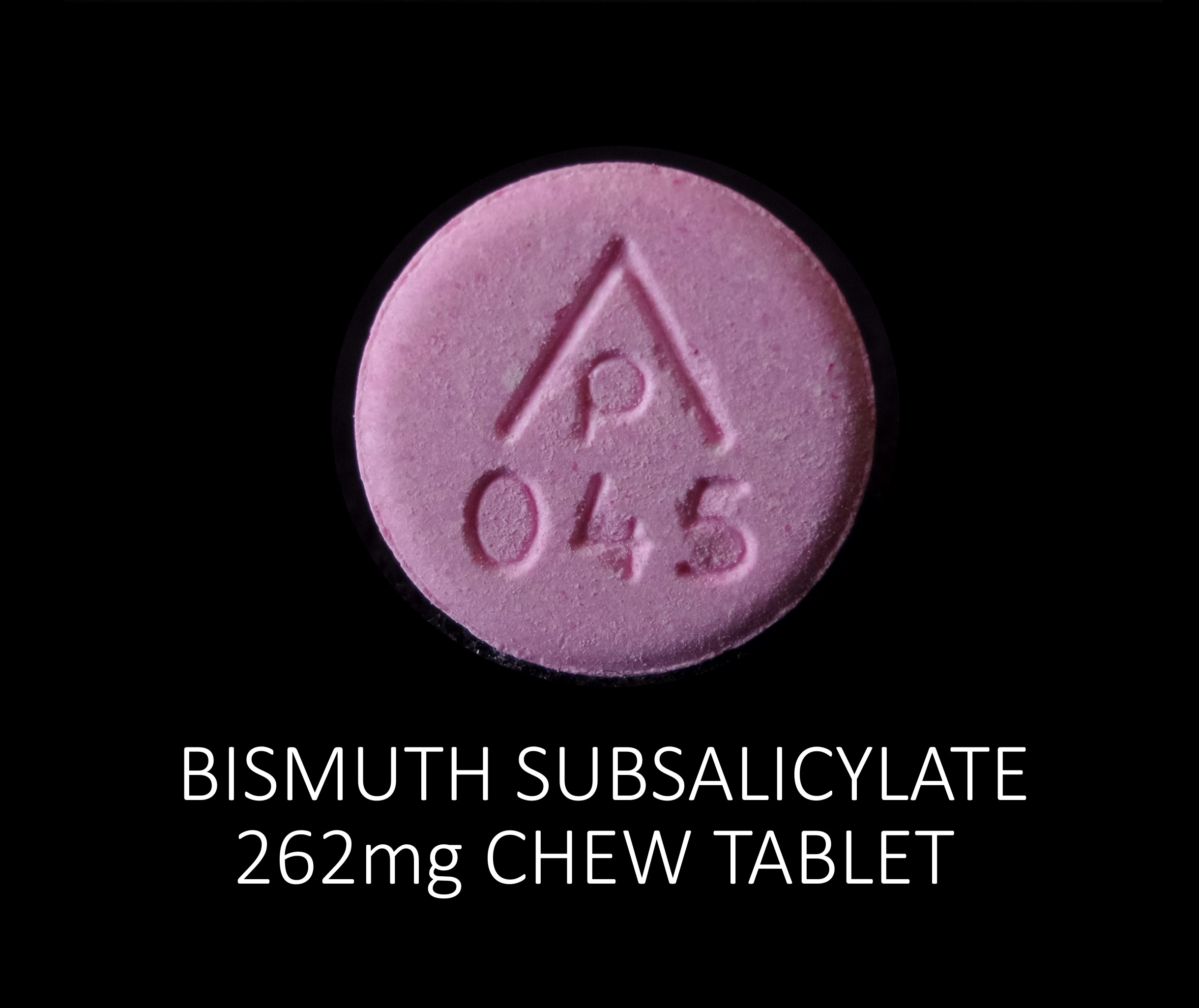
BISMUTH SUBSALICYLATE 262 MG- bismuth subsalicylate tablet, chewable
Preferred Pharmaceuticals Inc.
----------
Bismuth Subsalicylate 262 mg
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylates. Do not take if you are:
- allergic to salicylates (including aspirin)
- taking other salicylate products
Do not use
- if you have bloody or black stool
- if you have an ulcer or bleeding problem
Drug Facts continued on back of label
Ask a doctor or pharmacist before use if you are taking any drug for
- anticoagulation (thinning the blood)
- diabetes
- gout
- arthritis
Stop use and ask a doctor if
- symptoms get worse or last more than 2 days
- ringing in the ears or loss of hearing occurs
- diarrhea lasts more than 2 days
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- repeat dosage every 1/2 to 1 hour as needed
- do not take more than 8 doses in 24 hours
- use until diarrhea stops but not more than 2 days
- adults and children 12 years and over: 2 tablets
- children under 12 years: ask a doctor
Other information
- each tablet contains: calcium 77 mg, salicylate 102 mg
- store at 15°-30°C (59°-86°F)
- protect from moisture
Inactive ingredients
calcium carbonate, D&C red #27 (Al-lake), dextrose, flavor (cherry), magnesium stearate, maltodextrin, silicon dioxide, sorbitol
*Reliable 1 Laboratories LLC is not affiliated with the owner of the trademark Pepto-Bismol®
Distributed by: Reliable 1 Laboratories LLC, Valley Stream, NY 11580
Relabeled By: Preferred Pharmaceuticals Inc.
NDC: 68788-8128-3
Bismuth Subsalicylate
262 mg
UPSET STOMACH RELIEVER / ANTIDIARRHEAL
30 CHEWABLE TABLETS
*compare to Active Ingredients in Pepto-Bismol®
| BISMUTH SUBSALICYLATE 262 MG
bismuth subsalicylate tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | RELABEL(68788-8128) | |