ALBUMINAR-20 (albumin- human solution
Albuminar-20 by
Drug Labeling and Warnings
Albuminar-20 by is a Other medication manufactured, distributed, or labeled by CSL Behring LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
NDC: 0053-7695-90
20%
50 mL
Albumin (Human)
USP 20%
Albuminar®-20Store between 20-25°C (68-77°F);
excursions permitted to 15-30°C (59-86°F)
[See USP Controlled Room Temperature].Rx only
I.V. use onlyManufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767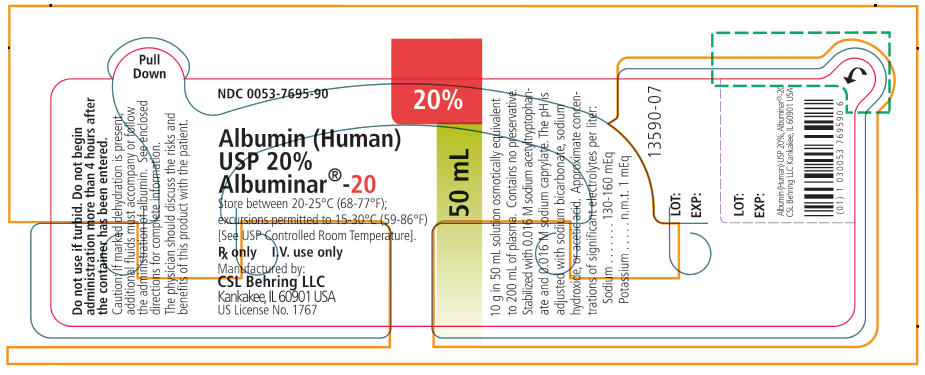
-
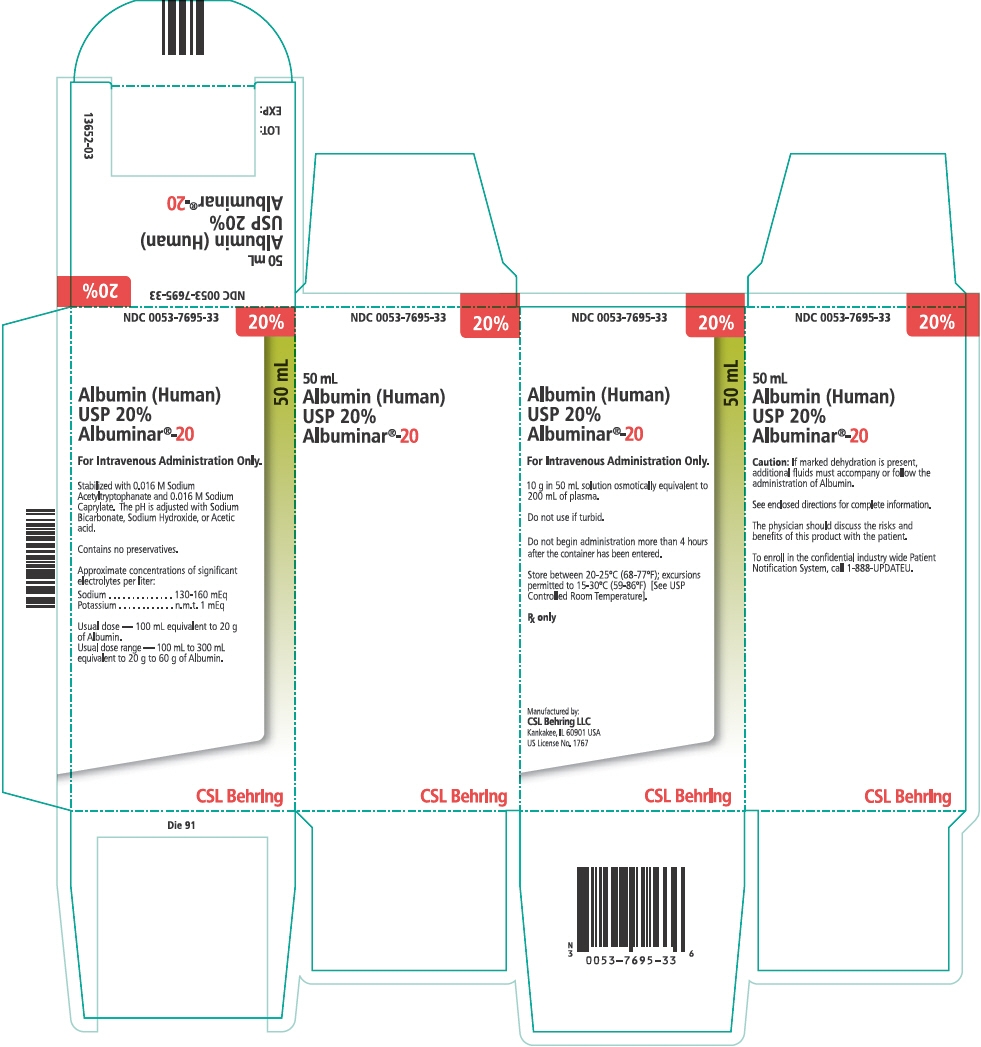
PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC: 0053-7695-33
20%
50 mL
Albumin (Human)
USP 20%
Albuminar®-20For Intravenous Administration Only.
10 g in 50 mL solution osmotically equivalent to
200 mL of plasma.Do not use if turbid.
Do not begin administration more than 4 hours
after the container has been entered.Store between 20-25°C (68-77°F); excursions
permitted to 15-30°C (59-86°F) [See USP
Controlled Room Temperature].Rx only
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767CSL Behring
-
INGREDIENTS AND APPEARANCE
ALBUMINAR-20
albumin (human) solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 0053-7695 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albumin Human (UNII: ZIF514RVZR) (Albumin Human - UNII:ZIF514RVZR) Albumin Human 20 g in 100 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) Sodium bicarbonate (UNII: 8MDF5V39QO) Sodium hydroxide (UNII: 55X04QC32I) Acetic Acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0053-7695-33 1 in 1 CARTON 1 NDC: 0053-7695-90 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 0053-7695-34 1 in 1 CARTON 2 NDC: 0053-7695-91 100 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/06/2009 09/07/2022 Labeler - CSL Behring LLC (058268293) Establishment Name Address ID/FEI Business Operations CSL Behring LLC 058268293 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.