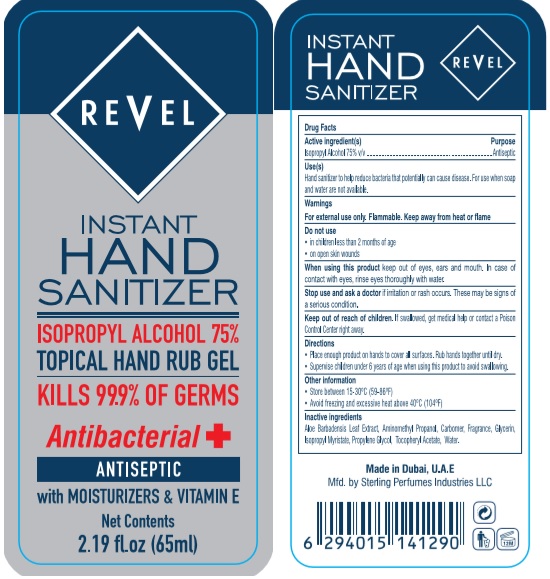
Revel Instant Hand Sanitizer (gel): (500 mL) NDC: 77716-015-16 (65 mL) NDC: 77716-01-02 (250 mL) NDC: 77716-015-25
Revel Instant Hand Sanitizer by
Drug Labeling and Warnings
Revel Instant Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Sterling Perfumes Industries LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REVEL INSTANT HAND SANITIZER- isopropyl alcohol gel
Sterling Perfumes Industries LLC
----------
Revel Instant Hand Sanitizer (gel):
(500 mL) NDC: 77716-015-16
(65 mL) NDC: 77716-01-02
(250 mL) NDC: 77716-015-25
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Inactive ingredients
Aloe Barbadensis Leaf Extract, Aminomethyl Propanol, Carbomer, Fragrance, Glycerin, Isopropyl Myristate, Propylene Glycol, Tocopheryl Acetate, Water.
Package Label - Principal Display Panel
Manufactured by Sterling Perfumes Industries, LLC
Revel Instant Hand Sanitizer (500 mL) NDC: 77716-015-16

Revel Instant Hand Sanitizer (65 mL) NDC: 77716-015-02
Revel Instant Hand Sanitizer (250 mL) NDC: 77716-015-25

| REVEL INSTANT HAND SANITIZER
isopropyl alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sterling Perfumes Industries LLC (561432456) |