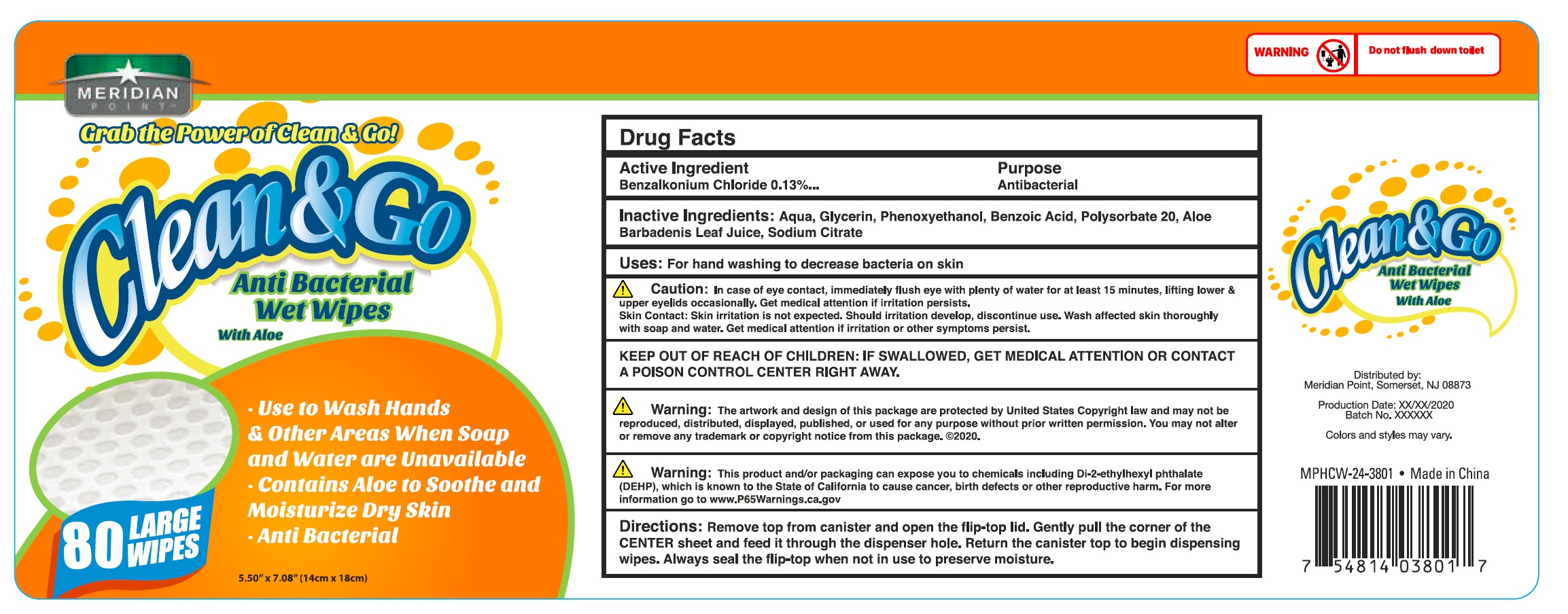
73760-005 Antibacterial Wet Wipes 0.13%Benzalkonium Chloride
Antibacterial Wet wipes by
Drug Labeling and Warnings
Antibacterial Wet wipes by is a Otc medication manufactured, distributed, or labeled by Zhejiang Easyclean Daily Chemical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL WET WIPES- benzalkonium chloride cloth
Zhejiang Easyclean Daily Chemical Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
73760-005 Antibacterial Wet Wipes 0.13%Benzalkonium Chloride
Warning
Warning: The artwork and design of this package are protected by United States Copyright law and may not be
reproduced, distributed, displayed, published, or used for any purpose without prior written permission. You may not alter
or remove any trademark or copyright notice from this package.⑥2020.
Warning: This product and/or packaging can expose you to chemicals including Di-2: -ethylhexyl phthalate
(DEHP), which is known to the State of California to cause cancer, birth defects or other reproductive harm. For more
information go to www.P65Warnings.ca.gov
Caution: In case of eye contact, immediately flush eye with plenty of water for at least 15 minutes, lifting lower &
upper eyelids occasionally. Get medical attention if irritation persists.
Skin Contact: Skin irritation is not expected. Should irritation develop, discontinue use. Wash affected skin thoroughly
with soap and water. Get medical attention if irritation or other symptoms persist.
Directions
Directions: Remove top from canister and open the flip-top lid. Gently pull the corner of the
CENTER sheet and feed it through the dispenser hole. Return the canister top to begin dispensing
wipes. Always seal the flip-top when not in use to preserve moisture.
| ANTIBACTERIAL WET WIPES
benzalkonium chloride cloth |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Zhejiang Easyclean Daily Chemical Co., Ltd. (554525882) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Easyclean Daily Chemical Co., Ltd. | 554525882 | manufacture(73760-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.