CARDAMYST- etripamil spray
Cardamyst by
Drug Labeling and Warnings
Cardamyst by is a Prescription medication manufactured, distributed, or labeled by Milestone Pharmaceuticals USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CARDAMYSTTM safely and effectively. See full prescribing information for CARDAMYSTTM.
CARDAMYSTTM (etripamil) nasal spray
Initial U.S. Approval: 2025INDICATIONS AND USAGE
CARDAMYST is a calcium channel blocker indicated for the conversion of acute symptomatic episodes of paroxysmal supraventricular tachycardia (PSVT) to sinus rhythm in adults (1).
DOSAGE AND ADMINISTRATION
- For intranasal use only (2.1).
- Initial dosage: A dose of 70 mg is administered as two nasal sprays, one spray into each nostril. Each nasal spray device delivers two sprays. The two sprays together contain a total of 70 mg etripamil (2.1).
- Repeat dosage (if needed): Should symptoms persist for 10 minutes after administration of CARDAMYST, take a second dose of 70 mg administered as two nasal sprays, one spray into each nostril. Do not exceed 140 mg in a 24-hour period (2.1).
DOSAGE FORMS AND STRENGTHS
Nasal spray: 70 mg etripamil per device (3).
CONTRAINDICATIONS
- Hypersensitivity to CARDAMYST or any of its components (4).
- Heart failure - New York Heart Association (NYHA) Class II to IV (4).
- Wolff-Parkinson-White (WPW), Lown-Ganong-Levine (LGL) syndromes, or manifest pre-excitation (delta wave) on a 12-lead ECG (4).
- Sick sinus syndrome (except in patients with a permanent pacemaker) (4)
- Second degree atrioventricular (AV) Mobitz 2 block or higher degree of AV block (4)
WARNINGS AND PRECAUTIONS
- Syncope: May cause dizziness and/or syncope, especially in patients with a history of syncope. Administer in a sitting position (5.1).
ADVERSE REACTIONS
Most common adverse reactions (incidence > 5%) are nasal discomfort, nasal congestion, rhinorrhea, throat irritation, and epistaxis (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Milestone Pharmaceuticals USA, INC. at toll-free phone 1-877-207-4764 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Lactation: A lactating woman should pump and discard breastmilk for 12 hours after CARDAMYST administration (8.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Syncope Related to Hemodynamic Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer as soon as possible after PSVT symptom onset.
Administer CARDAMYST by the nasal route only.
Each CARDAMYST device delivers two sprays for a total of 70 mg.
Recommended Dosage:
Using one nasal spray device, administer one spray into each nostril for a total initial dose of 70 mg. If symptoms persist after 10 minutes, use the second nasal spray device to administer a second dose of one spray into each nostril (70 mg total). Patients and caregivers should call their healthcare provider or seek emergency medical help if symptoms do not improve within 20 minutes after a second dose. Do not exceed 140 mg in a 24-hour period. See Instructions for Use for proper nasal spray technique.
If a full initial dose (i.e., 2 sprays, one in each nostril) is not administered due to device malfunction or misuse, the patient should wait at least 10 minutes before self-administering a second dose, if needed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
CARDAMYST is contraindicated in patients with:
- Hypersensitivity to CARDAMYST or any of its components.
- Heart failure – New York Heart Association (NYHA) Class II to IV.
- Wolff-Parkinson-White (WPW), Lown-Ganong-Levine (LGL) syndromes, or manifest pre-excitation (delta wave) on a 12-lead electrocardiogram (ECG).
- Sick sinus syndrome without a permanent pacemaker.
- Second degree atrioventricular (AV) Mobitz 2 block or higher degree of AV block.
-
5 WARNINGS AND PRECAUTIONS
5.1 Syncope Related to Hemodynamic Effects
Because of effects on blood pressure, heart rate, and cardiac conduction, CARDAMYST may cause dizziness and/or syncope, especially in patients with a history of syncope and high-grade AV block or sinus node dysfunction, or those with a history of syncope during an episode of PSVT. In clinical trials, a small percentage of patients (0.4%) experienced clinically significant hypotension during test dosing prior to randomization, which precluded further participation in the study. Patients with a history of hypotensive episodes or those at increased risk for hemodynamic instability should be monitored appropriately when initiating CARDAMYST.
If syncope occurs, patients should be placed in the recumbent position and treated supportively.
Patients should be cautioned about these possible adverse effects and advised to administer CARDAMYST in a sitting position, and in a location where the risk of fall is minimal.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of syncope [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of CARDAMYST was evaluated using pooled data from double-blind, randomized, placebo-controlled trials including NODE-1, NODE-301 Part 1, RAPID, and RAPID Extension. A total of 321 patients were treated with CARDAMYST in randomized controlled studies.
In the RAPID and RAPID Extension studies, in which patients had the option of self-administering a second dose of CARDAMYST for a perceived episode of PSVT, the majority of patients (65%) self-administered a second dose of CARDAMYST (2x70mg).
In NODE-301 Part 1, RAPID, and RAPID Extension, to assess tolerability, a test dose(s) was given prior to randomization. A small percentage of patients failed the test dose due to hypotension (0.4%) [see Warnings and Precautions (5.1)].
The majority of treatment-related adverse reactions reported in clinical studies with CARDAMYST have been related to local reactions to, at, or near the nasal administration site, including the nose, throat, and eyes. These local reactions included nasal discomfort, nasal congestion, throat irritation, oropharyngeal pain, lacrimation, rhinorrhea, bleeding from the nose, upper-airway cough syndrome, and sneezing.
Table 1: Most frequent (≥5.0%) Adverse Reactions1 Observed in Randomized Controlled Studies 1) Adverse reactions that occurred within 24 hours of study drug administration (TEAE24h) for perceived PSVT in the double-blind, placebo-controlled studies, NODE-1, NODE-301 Part 1, RAPID and RAPID Extension that had an overall incidence of 5% or greater and where the incidence is at least 1% greater than the placebo group.
2) 2x70 mg: first administration of etripamil 70 mg followed by a second dose of etripamil 70 mg 10 minutes later if symptoms persisted.
Placebo
N=223
%CARDAMYST 70 mg
N=235
%CARDAMYST 2x70 mg2
N=86
%Nasal Discomfort 6 28 23 Nasal Congestion 1 14 12 Rhinorrhea 2 12 10 Throat Irritation 1 7 6 Epistaxis 1 6 7 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on the use of CARDAMYST during pregnancy to inform a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Reproductive studies conducted with intravenous administration of etripamil in pregnant rats and rabbits during organogenesis did not show any evidence of fetal harm or malformations in rats at exposures up to approximately 3x the maximum concentration (Cmax) and 0.4x the AUC at the maximum recommended human dose (MRHD) and in rabbits at exposures approximately equivalent to the Cmax and 10x the AUC at the MRHD, at which maternal toxicities were observed (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In pregnant rats, intravenous administration of etripamil throughout the period of organogenesis did not result in any adverse effects on embryofetal development at doses up to 0.375 mg/kg/day, approximately 3x the Cmax and 0.4x the AUC at the MRHD.
In pregnant rabbits, intravenous administration of etripamil throughout the period of organogenesis did not result in embryofetal abnormalities at doses up to 0.1 mg/kg/day, approximately equivalent to the Cmax and 10x the AUC at the MRHD. Abortion in one animal was noted at the high dose of 0.1 mg/kg/day, a dose that caused maternal toxicity.
In the pre- and post-natal toxicity study in rats, intravenous administration of etripamil from gestation day 7 through the lactation period (post-partum day 20), did not show any adverse effects on pre- and postnatal development at doses up to 0.374 mg/kg/day, approximately 3x the Cmax and 0.4x the AUC at the MRHD. Post-implantation loss was noted at 0.374 mg/kg/day, a dose that also caused significant maternal toxicity, including mortality, transient adverse clinical signs, and body weight reduction.
8.2 Lactation
Risk Summary
There are no data on the presence of etripamil in human milk or animal milk. However, the structurally related compound, verapamil, is known to be present in human milk. There are no data on the effects of etripamil on the breastfed infant or on milk production. Because the presence of etripamil in breastmilk has not been characterized, and there is a potential for adverse reactions in the breastfed infant including hypotension and bradycardia, lactating women should interrupt breastfeeding and pump and discard milk for 12 hours (approximately 5 terminal half-lives) after treatment with CARDAMYST.
8.4 Pediatric Use
The safety and effectiveness of CARDAMYST have not been established in the pediatric population.
Etripamil is structurally similar to another drug in the same pharmacologic class that has been associated with a high risk of potentially non-reversible electromechanical dissociation or cardiovascular collapse in pediatric patients less than 1 year of age, including neonates.
-
10 OVERDOSAGE
Overdosage is expected to cause peripheral vasodilation with possible symptomatic hypotension and reflex tachycardia. AV block and /or pauses may also occur.
Treatment of overdosage should be supportive. Beta-adrenergic stimulation or parenteral administration of calcium solutions may increase calcium ion flux across the slow calcium channel. Clinically significant hypotensive reactions or high degree AV block should be treated with fluid administration or vasopressor agents, or cardiac pacing, respectively. Asystole should be handled by the usual measures including cardiopulmonary resuscitation.
It is unknown whether etripamil is dialyzable. However, the structurally related compound, verapamil cannot be removed by hemodialysis.
-
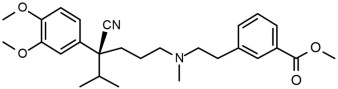
11 DESCRIPTION
Etripamil, the active ingredient of CARDAMYST is a calcium channel blocker.
The chemical name of etripamil is benzoic acid, 3-[2-[[(4S)-4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl]methylamino]ethyl]-, methyl ester.
Its molecular weight is 452.59 and its molecular formula is C27H36N2O4.
The structural formula is:
Etripamil is a colorless to slightly yellow oil. Etripamil has a pKa of 8.57 and is very soluble in methyl tert-butyl ether, freely soluble in methanol, dichloromethane and acetone, sparingly soluble in ethanol and hexane, and insoluble in water.
CARDAMYST is a spray intended for nasal administration. Each device of CARDAMYST delivers two metered sprays of etripamil with a total of 70 mg. CARDAMYST contains 350 mg/mL of etripamil and the following inactive ingredients: acetic acid, edetate disodium, sulfuric acid for pH adjustment, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Etripamil is an L-type calcium influx inhibitor (slow channel blocker or calcium ion antagonist). Etripamil exerts its pharmacologic effect by modulating the influx of ionic calcium across the cell membrane of the AV nodal cells as well as arterial smooth muscles and contractile myocardial cells. By interrupting reentry at the AV node, etripamil can restore sinus rhythm in patients with PSVT.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Etripamil prolonged the baseline PR interval by 8% to 10% approximately 5 minutes following an intranasal dose of 70 mg. This PR interval prolongation begins to decline shortly after the etripamil maximum concentration (Cmax) and falls below the 8 to 10% threshold between 25 and 50 minutes. Etripamil mean maximum change from baseline PR interval was approximately 13% following a single 70 mg dose and 20% following two 70 mg doses in the NODE-103 trial. At a dose of 1 to 2 times the maximum recommended therapeutic dose, etripamil does not prolong the QTc interval.
Hemodynamics
In patients with induced supraventricular tachycardia in an electrophysiology laboratory, a single intranasal etripamil administration was associated with a maximal systolic blood pressure reduction of approximately 21 mm Hg with 140 mg (2 x maximum approved dose), 17 mm Hg with 105 mg (1.5 x maximum approved dose), and 3 mm Hg with 70 mg dose. No reduction in blood pressure was observed with etripamil 35 mg dose.
12.3 Pharmacokinetics
After one 70 mg dose of etripamil, mean (%CV) area under the concentration-time curve (AUC) is approximately 5461 (51.6%) ng*min/mL and the Cmax is approximately 99 (64.6%) ng/mL. After a second 70 mg dose of etripamil administered 10 minutes after the first dose, mean (%CV) AUC is approximately 7721 (50.3%) ng*min/mL and the Cmax is approximately 132 (59.1%) ng/mL.
Absorption
Etripamil median (range) time to Cmax (Tmax) is 7 minutes (3 to 20 minutes) following a single intranasal administration of 70 mg. Median Tmax is 13 minutes (3 to 35 minutes) following a second intranasal administration of 70 mg.
Distribution
Etripamil mean apparent volume of distribution ranges from approximately 2200 to 3500 L. Etripamil plasma protein binding is approximately 50%.
Elimination
Average etripamil concentration fell by approximately 60% of its peak value (Cmax) at 25 minutes and 80% of the Cmax by 60 minutes after dosing. Subsequently, concentrations decrease at a slower rate, and this decline is associated with a half-life of approximately 2.5 hours.
Metabolism: Etripamil metabolic pathways include hydrolysis, demethylation, N-dealkylation, and secondary oxidation, glucuronidation, and taurine conjugation. Etripamil is primarily metabolized by blood esterases and hepatic metabolism, primarily via CYP3A4, and CYP3A5. Etripamil contains a methyl ester which renders it metabolically sensitive to bloodborne esterases.
Excretion: After a single dose of radiolabeled intranasal etripamil 70 mg to healthy subjects, approximately 29% of the dose was recovered in urine (<0.05% unchanged), 26% was recovered in feces (<0.05% unchanged), and the remainder was recovered on nose and face tissues. Approximately 71% of the total administered dose was recovered in 7-10 days.
Specific Populations
No clinically significant differences in the pharmacokinetics of etripamil were observed based on age (19 to 56 years old), body weight (49 to 91 kg), height (157 to 194 cm), sex, or race (Caucasian, Asian, or African American). The effect of renal impairment (eGFR < 90 mL/min) or hepatic impairment (Child Pugh A, B, or C) on etripamil pharmacokinetics is unknown. It is unknown whether etripamil is dialyzable.
Drug Interaction Studies
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Etripamil was not genotoxic in a bacterial reverse mutation assay, an in vitro chromosome aberration assay in human peripheral lymphocytes, and an in vivo micronucleus study in rats (IV administration).
Impairment of Fertility
In a male and female fertility study, rats were dosed once daily via intravenous administration at etripamil doses of 0.05 to 0.374 mg/kg/day. Male rats were dosed for 28 days prior to mating, and female rats were dosed for 14 days prior to mating and continuing up to day 7 of pregnancy. There was no effect on fertility of male and female rats at doses up to 0.374 mg/kg/day, approximately 3× the Cmax and 0.4× the AUC at the MRHD.
-
14 CLINICAL STUDIES
The RAPID study (NCT #03464019) was a randomized, double-blind, placebo-controlled, multicenter, event-driven, phase 3 study designed to evaluate the efficacy and safety of CARDAMYST in patients with a history of symptomatic PSVT. Six hundred ninety-two (692) patients were randomized 1:1 to CARDAMYST 70 mg or placebo. Patients with an episode of perceived PSVT were to self-administer the study drug intranasally in a medically unsupervised setting and self-administer a second dose of the study drug if symptoms persisted at 10 minutes after the first dose. Continuously obtained electrocardiographic data during the episode of PSVT were blindly adjudicated. The primary endpoint was time-to-conversion of confirmed PSVT to sinus rhythm for at least 30 seconds within 30 minutes of the first dose.
Of the 692 randomized patients, 255 patients perceived an episode of PSVT and self-administered the study drug; 184 (72%) episodes were confirmed by blinded adjudication to be PSVT. Patients had a median age of 54 years (range 19 to 78 years) and were 71% female, 93% Caucasian, 3% Black and 4% other. Sixty-three percent (63%) of patients with confirmed PSVT episode were taking concomitant beta blocker or calcium channel blocker.
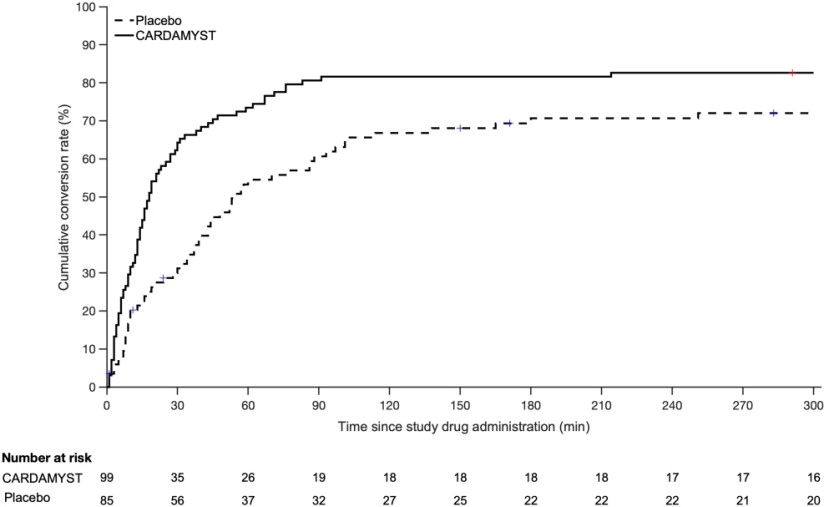
In the study's primary analysis, patients with confirmed episodes of PSVT, Kaplan-Meier Estimates of those who converted to sinus rhythm within 30 minutes were 64% and 31% for CARDAMYST and placebo, respectively, with a hazard ratio of 2.6 [95% CI: (1.7, 4.2)], p-value <0.001. Median time-to-conversion was 17.2 minutes (95% CI: 13.4, 26.5) with CARDAMYST versus 53.5 minutes (95% CI: 38.7, 87.3) with placebo. Kaplan-Meier estimates of conversion remained in favor of CARDAMYST at 300 minutes; hazard ratio 1.7 [95% CI: (1.2, 2.4)].
Kaplan-Meier plot of estimated probabilities of achieving sinus rhythm following treatment with CARDAMYST are shown in Figure 1.
Figure 1: RAPID Primary Efficacy Outcome Kaplan Meier Curve: Conversion to Sinus Rhythm in the Patient Population with Confirmed PSVT1
1) 71/255 (28%) patients who self-administered CARDAMYST for a perceived episode of PSVT did not have confirmed PSVT and are excluded.
Seventy-one (71) of 255 (28%) patients who self-administered CARDAMYST for a perceived episode of PSVT did not have PSVT confirmed due to missing ECG data (4%), resolution of PSVT prior to dosing (5%), or other rhythm diagnoses (19%). In an analysis assuming all 71 of these patients did not convert, the Kaplan-Meier estimates for conversion to sinus rhythm within 30 minutes were 50% and 23% for the CARDAMYST and placebo groups, respectively, with a hazard ratio of 2.6 [95% CI: (1.6, 4.1)].
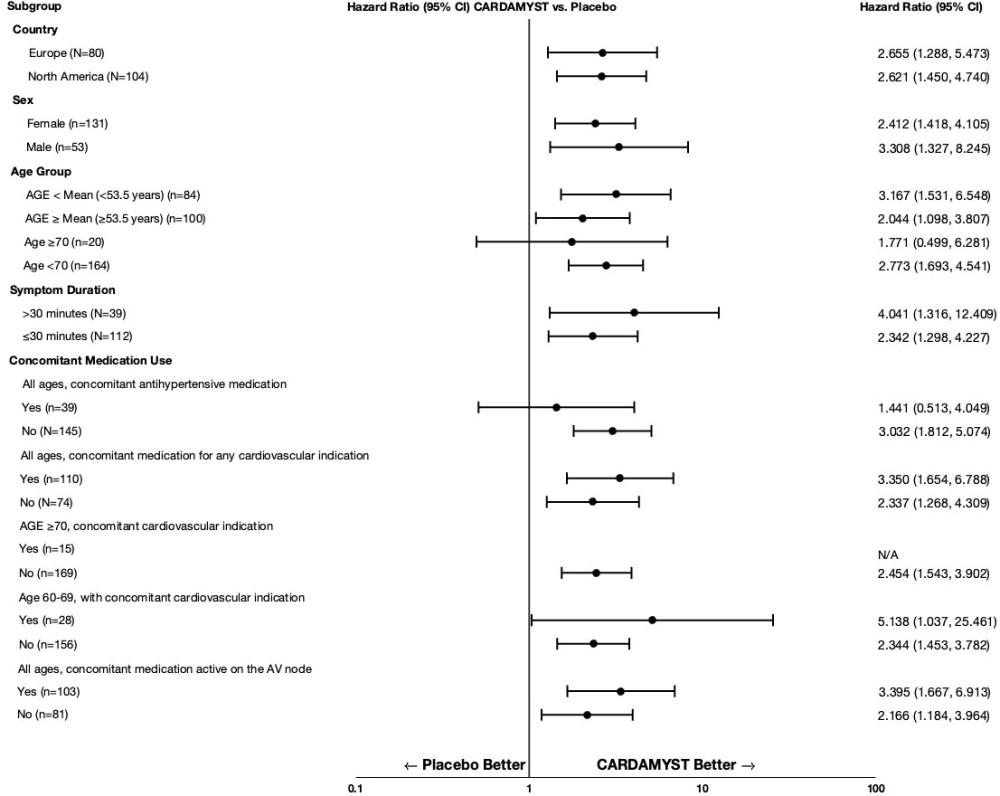
In RAPID, the results for the primary efficacy endpoint were generally consistent across major subgroups including geographic region, sex, age group, concomitant medications, and duration from onset of symptoms to first dose (Figure 2).
Figure 2: Conversion to Sinus Rhythm at 30 Minutes Hazard Ratios by Baseline Characteristics - RAPID Study
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
CARDAMYST is supplied in cartons (NDC: 83468-070-03) of 2 disposable nasal spray devices contained in a plastic carrying case.
Each nasal spray device delivers two sprays containing a total of 70 mg etripamil.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Important Treatment Instructions
Instruct patients and caregivers on PSVT symptoms and the timing of administration in relation to the onset of the episode of PSVT. Instruct patients and caregivers on what to observe following administration, and what would constitute an outcome requiring medical attention. Instruct patients and caregivers to call their healthcare provider or seek emergency medical help if symptoms do not improve within 20 minutes after a second dose.
Administration Information
Instruct patients and caregivers not to test or prime before use. Instruct patients and caregivers to discard the CARDAMYST device after use.
Lactation
Advise lactating women to pump and discard breastmilk for 12 hours after CARDAMYST administration in order to minimize exposure to a breastfed infant [see Use in Specific Populations (8.2)].
Manufactured for: Milestone Pharmaceuticals USA, Inc. 6210 Ardrey Kell Road, Suite 650, Charlotte, NC, 28277
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: December 2025
PATIENT INFORMATION
CARDAMYST™ (kar da mist)
(etripamil)
nasal sprayWhat is CARDAMYST?
CARDAMYST is a prescription medicine used to help restore normal heart rhythm in adults who have symptoms of sudden episodes of fast heartbeat called paroxysmal supraventricular tachycardia (PSVT).
It is not known if CARDAMYST is safe and effective in children.Do not use CARDAMYST if you:
- are allergic to CARDAMYST or to any of its ingredients. See the end of this leaflet for a complete list of ingredients in CARDAMYST.
- have limitations in activities due to heart failure (moderate to severe heart failure).
- have Wolff-Parkinson-White (WPW) syndrome, Lown-Ganong-Levine (LGL) syndrome, or an abnormal heart rhythm pattern called pre-excitation (delta wave) on an electrocardiogram (ECG).
- have sick sinus syndrome without a permanent pacemaker.
- have second degree or higher atrioventricular (AV) block.
Before using CARDAMYST, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of fainting.
- have low blood pressure.
- are pregnant or plan to become pregnant. It is not known if CARDAMYST will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if CARDAMYST passes into your breast milk. You should stop breastfeeding for 12 hours after treatment with CARDAMYST. During this time, pump and throw away your breast milk. Talk to your healthcare provider about the best way to feed your baby after using CARDAMYST.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How should I use CARDAMYST?
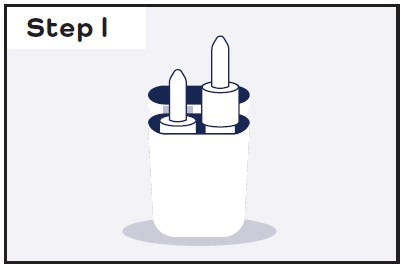
- Read the Instructions for Use that comes with CARDAMYST for information about the right way to use CARDAMYST.
- Use CARDAMYST exactly as prescribed by your healthcare provider.
- CARDAMYST is for use in the nose only.
- Each CARDAMYST device contains 1 dose consisting of 2 sprays (1 spray in each nostril).
- Do not test, prime, or press the plunger before use. The nasal spray device is ready for use.
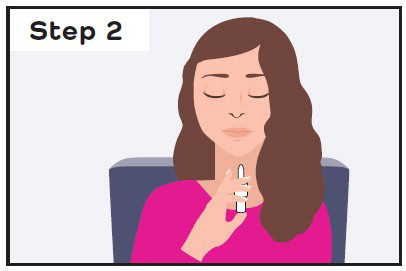
- Use CARDAMYST as soon as possible after your PSVT symptoms begin.
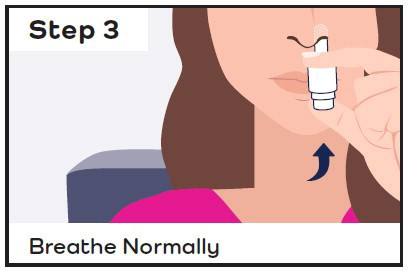
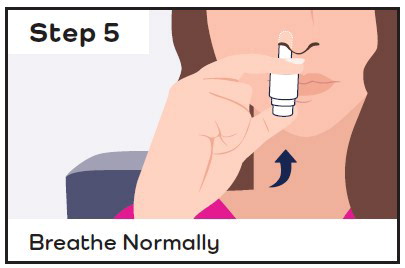
- While sitting, insert the CARDAMYST device into the nostril and spray one spray in each nostril by pressing the plunger firmly and quickly all the way up and releasing it between each spray.
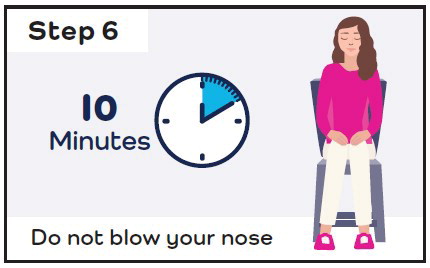
- Stay seated and keep your head straight for 10 minutes.
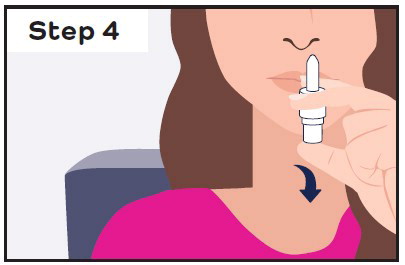
- If you still have symptoms after 10 minutes, give a second dose (1 spray in each nostril) with a new CARDAMYST device.
- If your symptoms do not improve within 20 minutes after your second dose, call your healthcare provider or get emergency medical help right away.
- If you do not receive the full first dose (1 spray in each nostril) because the device did not work or was used incorrectly, wait at least 10 minutes before giving a second dose, if needed.
- Do not use more than 2 CARDAMYST devices (140 mg) within a 24-hour period.
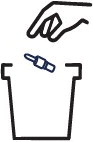
- Do not re-use the CARDAMYST device. Throw away (discard) the CARDAMYST device after use.
- If you use too much CARDAMYST, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of CARDAMYST?
CARDAMYST may cause serious side effects, including:
- Fainting due to CARDAMYST effects on blood pressure, heart rate, and electrical activity of the heart. CARDAMYST may cause dizziness and fainting, especially in people with a history of fainting and certain heart problems, or people with a history of fainting during an episode of PVST. Use CARDAMYST while sitting in a safe area where you will not fall if you become dizzy or lightheaded. Lie down if you feel dizzy or lightheaded after using CARDAMYST. If fainting occurs after using CARDAMYST, caregivers should place you on your back and seek medical help.
The most common side effects of CARDAMYST include: - nasal discomfort
- nasal congestion
- runny nose
- throat irritation
- nosebleed
These are not all of the possible side effects of CARDAMYST.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store CARDAMYST?
- Store CARDAMYST at room temperature between 68°F to 77°F (20°C to 25°C).
Keep CARDAMYST and all medicines out of the reach of children. General information about the safe and effective use of CARDAMYST.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CARDAMYST for a condition for which it was not prescribed. Do not give CARDAMYST to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about CARDAMYST that is written for health professionals.What are the ingredients in CARDAMYST?
Active ingredient: etripamil
Inactive ingredients: acetic acid, edetate disodium, sulfuric acid and water for injection.
Manufactured for: Milestone Pharmaceuticals USA, Inc., 6210 Ardrey Kell Road, Suite 650, Charlotte, NC, 28277
For more information about CARDAMYST, visit cardamyst.com or call 1-844-805-5810. -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
Cardamyst™ [kar da mist]
(etripamil) nasal spray
This Instructions for Use contains information on how to use CARDAMYST nasal spray.
Storing CARDAMYST
Store CARDAMYST at room temperature between 20°C to 25°C (68°F to 77°F).
Keep CARDAMYST and all medicines out of the reach of children. For more information about CARDAMYST, visit cardamyst.com or call 1-844-805-5810.
Manufactured for: Milestone Pharmaceuticals USA, Inc., 6210 Ardrey Kell Road, Suite 650, Charlotte, NC 28277
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: December 2025
-
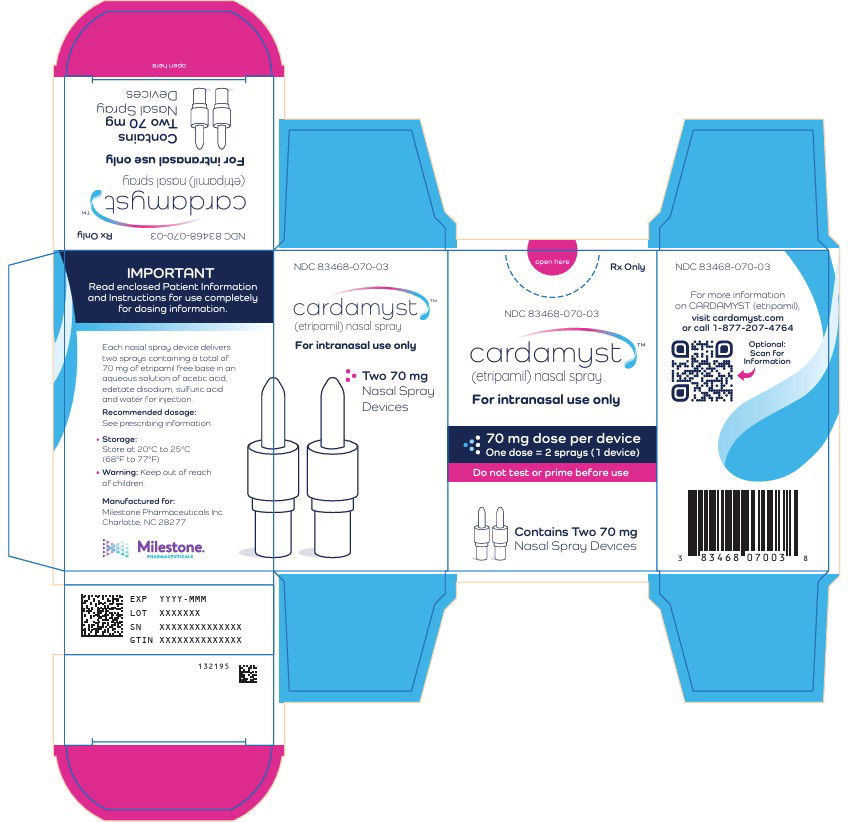
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 70 mg Carton Label
open here
Rx Only
NDC: 83468-070-03
cardamyst™
(etripamil) nasal sprayFor intranasal use only
70 mg dose per device
One dose = 2 sprays (1 device)Do not test or prime before use
Contains Two 70 mg
Nasal Spray Devices -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 70 mg Device Label
NDC: 83468-070-01 Rx Only
cardamyst™
(etripamil) nasal sprayFor intranasal use only
70 mg per Device
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 70 mg Container Label
NDC: 83468-070-02 Rx Only
cardamyst™
(etripamil) nasal sprayFor intranasal use only
70 mg dose per device
One dose = 2 sprays (1 device)Do not test or prime before use
Two 70 mg Nasal Spray Devices
Each device delivers two sprays
containing a total of 70 mg etripamil
Recommended dosage:
See prescribing informationImportant: Before
using, read enclosed
Instructions For Use -
INGREDIENTS AND APPEARANCE
CARDAMYST
etripamil sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83468-070 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength etripamil (UNII: S82A18Y42P) (etripamil - UNII:S82A18Y42P) etripamil 70 mg in 0.2 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) sulfuric acid (UNII: O40UQP6WCF) edetate disodium (UNII: 7FLD91C86K) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83468-070-03 2 in 1 CARTON 01/05/2026 1 NDC: 83468-070-02 2 in 1 CONTAINER 1 NDC: 83468-070-01 0.2 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218571 01/05/2026 Labeler - Milestone Pharmaceuticals USA, Inc. (117076001)
Trademark Results [Cardamyst]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CARDAMYST 98080988 not registered Live/Pending |
Milestone Pharmaceuticals USA, Inc. 2023-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.