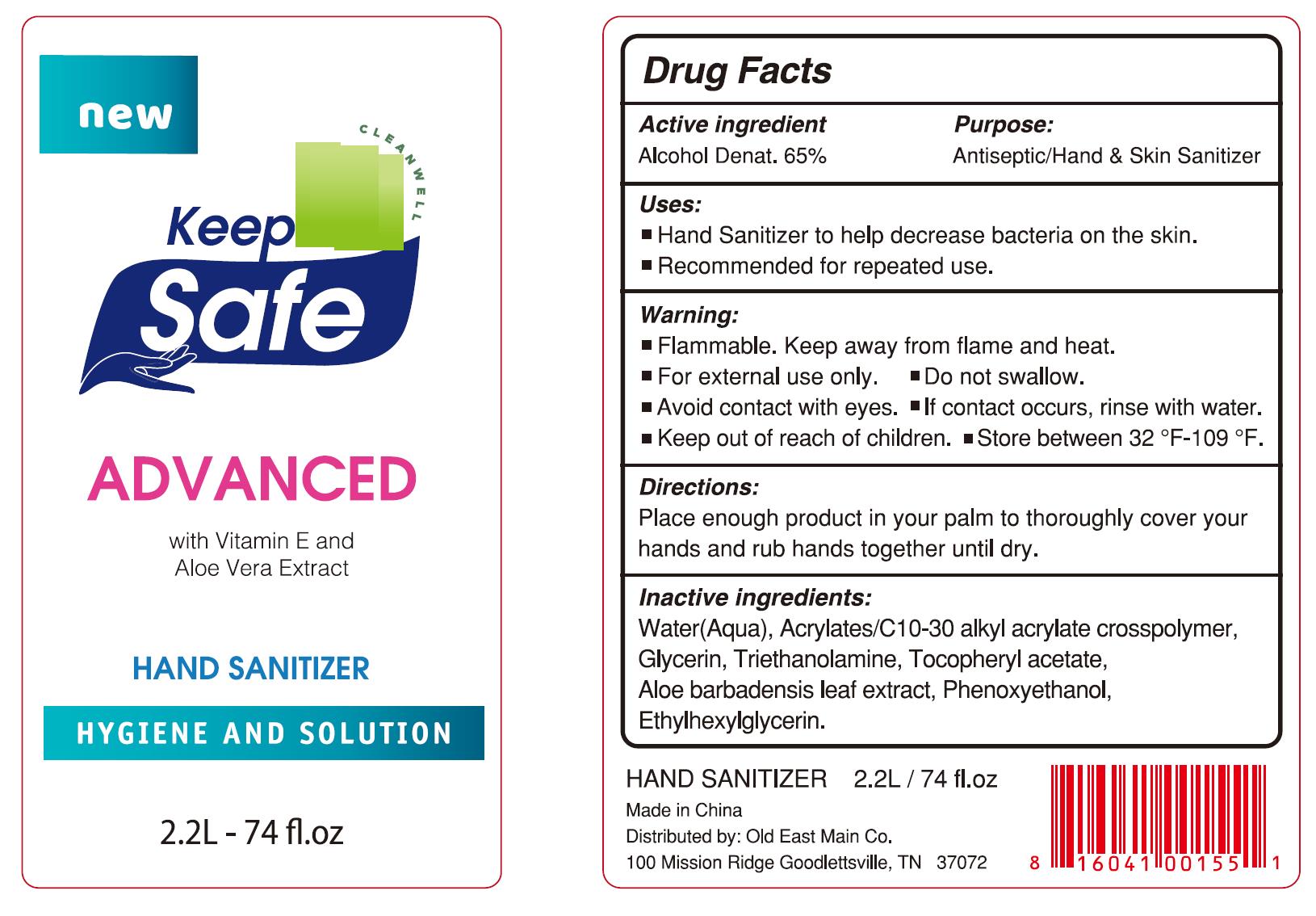
51706-910 Hand Sanitizer Ethyl Alcohol(65%)
Hand Sanitizer by
Drug Labeling and Warnings
Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Landy International, DOLLAR GENERAL CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HAND SANITIZER- ethyl alcohol gel
Landy International
----------
51706-910 Hand Sanitizer Ethyl Alcohol(65%)
Warnings
Flammable. Keep away from flame and heat.
For external use only.
Do not swallow.
Avoid contact with eyes. If contact occurs, rinse with water.
Keep out of reach of children.
Store between 32 °F-109 °F.
Directions
Place enough product in your palm to thoroughly cover your hands and rub
hands together until dry.
| HAND SANITIZER
ethyl alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Landy International (545291775) |
| Registrant - Landy International (545291775) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DOLLAR GENERAL CORPORATION | 006946172 | label(51706-910) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Landy International | 545291775 | manufacture(51706-910) | |
Revised: 10/2024
Document Id: 23ccb954-9be4-810c-e063-6394a90a5125
Set id: a9381981-b53c-1736-e053-2a95a90ae15c
Version: 3
Effective Time: 20241006
Trademark Results [Hand Sanitizer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HAND SANITIZER 88958909 not registered Live/Pending |
MAISON BLANCHE, LLC 2020-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.