Antibacterial Hand Sanitizer by Shanghai Xiaoguo New Material Technology Co., Ltd
Antibacterial Hand Sanitizer by
Drug Labeling and Warnings
Antibacterial Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Shanghai Xiaoguo New Material Technology Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL HAND SANITIZER- dbdmh liquid
Shanghai Xiaoguo New Material Technology Co., Ltd
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
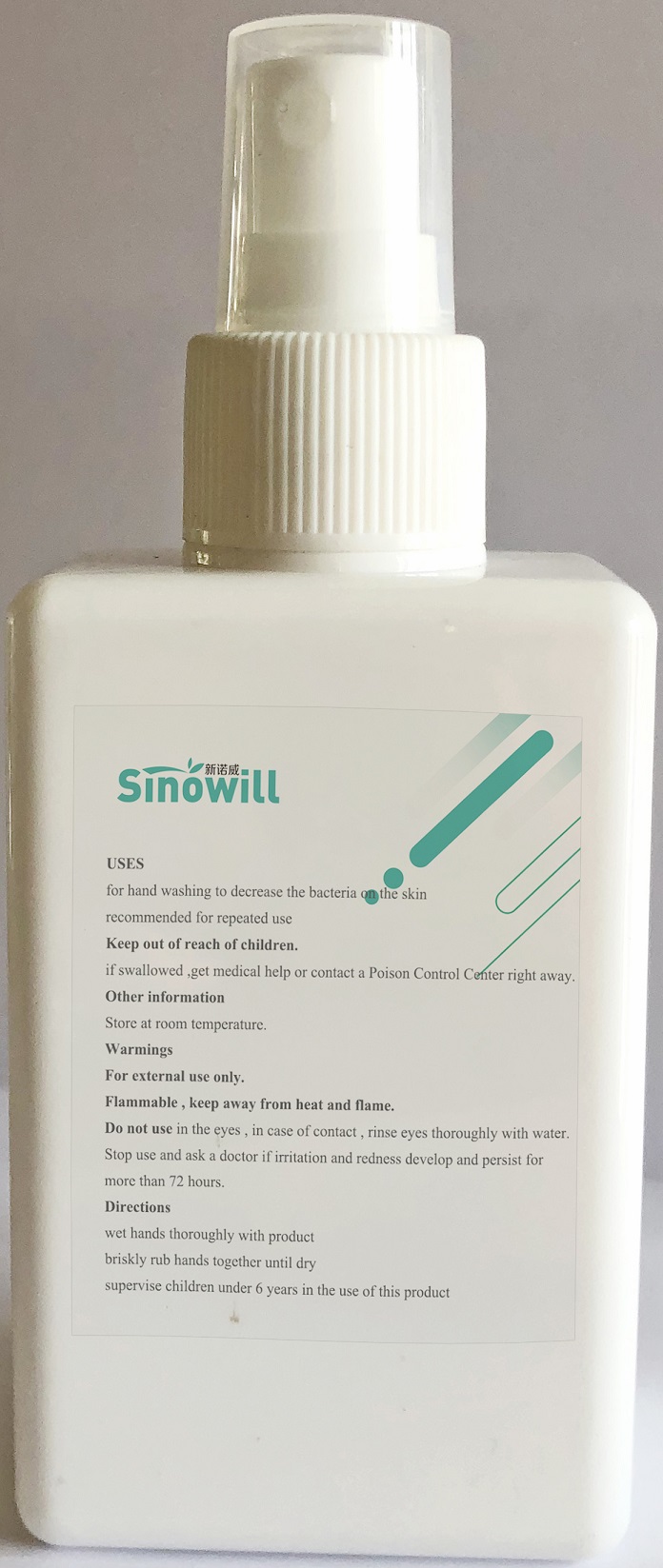
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| ANTIBACTERIAL HAND SANITIZER
dbdmh liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Shanghai Xiaoguo New Material Technology Co., Ltd (416844630) |
| Registrant - Shanghai Xiaoguo New Material Technology Co., Ltd (416844630) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai Xiaoguo New Material Technology Co., Ltd | 416844630 | manufacture(79277-001) | |
Revised: 1/2021
Document Id: b846f1dd-760d-c881-e053-2a95a90ae6e7
Set id: a94378ba-9acd-2e18-e053-2995a90a7c27
Version: 3
Effective Time: 20210106
Shanghai
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.