FORMULATION R- phenylephrine hydrochloride, hydrogenate palm kernel oil suppository
Formulation R by
Drug Labeling and Warnings
Formulation R by is a Otc medication manufactured, distributed, or labeled by G&W Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
USES
helps relieve the local itching and discomfort associated with hemorrhoids
temporarily relieves burning and shrinks hemorrhoidal tissue
temporarily provides a coating for relief of anorectal discomforts
temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful -
WARNINGS
For rectal use only
Ask a doctor before use if you have
heart disease
high blood pressure
thyroid disease
diabetes
difficulty in urination due to an enlarged prostate
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression
When using this product
do not use more than directed
Stop use and ask a doctor if
symptoms get worse or do not get better in 7 days
bleeding occurs - IF PREGNANT OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
adults: when practical, clean the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with tissue or a soft cloth before insertion of the product.
remove wrapper before inserting into the rectum as follows:
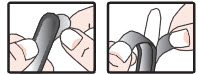
hold suppository with pointed end up
carefully separate tabs with fingernail
slowly pull apart by pulling tabs down on both sides
remove suppository from wrapper
insert one suppository into the rectum up to 4 times daily, especially at night, in the morning or after each bowel movement
children under 12 years: ask a doctor - OTHER INFORMATION
- INACTIVE INGREDIENT
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 0713-0535-24
G&W Formulation RTM HEMORRHOIDAL SUPPOSITORIES
Prompt Soothing Relief from Painful Burning, Itching and Discomfort
24 ADULT SUPPOSITORIES
Tamper-Evident: For your safety suppositories are packaged in "Tamper-Evident" sealed foil. Do not use if foil is torn or open
Compare to active ingredients in Preparation-H® Hemorrhoidal Suppositories
FOR TEMPORARY RELIEF FROM PAIN AND ITCHING OF HEMORRHOIDS
-
INGREDIENTS AND APPEARANCE
FORMULATION R
phenylephrine hydrochloride, hydrogenate palm kernel oil suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0713-0535 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Phenylephrine Hydrochloride (UNII: 04JA59TNSJ) (Phenylephrine - UNII:1WS297W6MV) Phenylephrine Hydrochloride 2.5 mg Hydrogenated Palm Kernel Oil (UNII: FM8D1RE2VP) (Hydrogenated Palm Kernel Oil - UNII:FM8D1RE2VP) Hydrogenated Palm Kernel Oil 855 mg Inactive Ingredients Ingredient Name Strength Cod Liver Oil (UNII: BBL281NWFG) Starch, Corn (UNII: O8232NY3SJ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0713-0535-24 24 in 1 CARTON 2 NDC: 0713-0535-12 12 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 06/24/2002 Labeler - G&W Laboratories, Inc. (001271188) Registrant - G&W Laboratories, Inc. (001271188) Establishment Name Address ID/FEI Business Operations G&W Laboratories, Inc. 001271188 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.