Puricia Sanitizing Wipes by PHARMABERG INC. Hand Sanitizing Wipes
Puricia Sanitizing Wipes by
Drug Labeling and Warnings
Puricia Sanitizing Wipes by is a Otc medication manufactured, distributed, or labeled by PHARMABERG INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PURICIA SANITIZING WIPES- ethyl alcohol 75% cloth
PHARMABERG INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
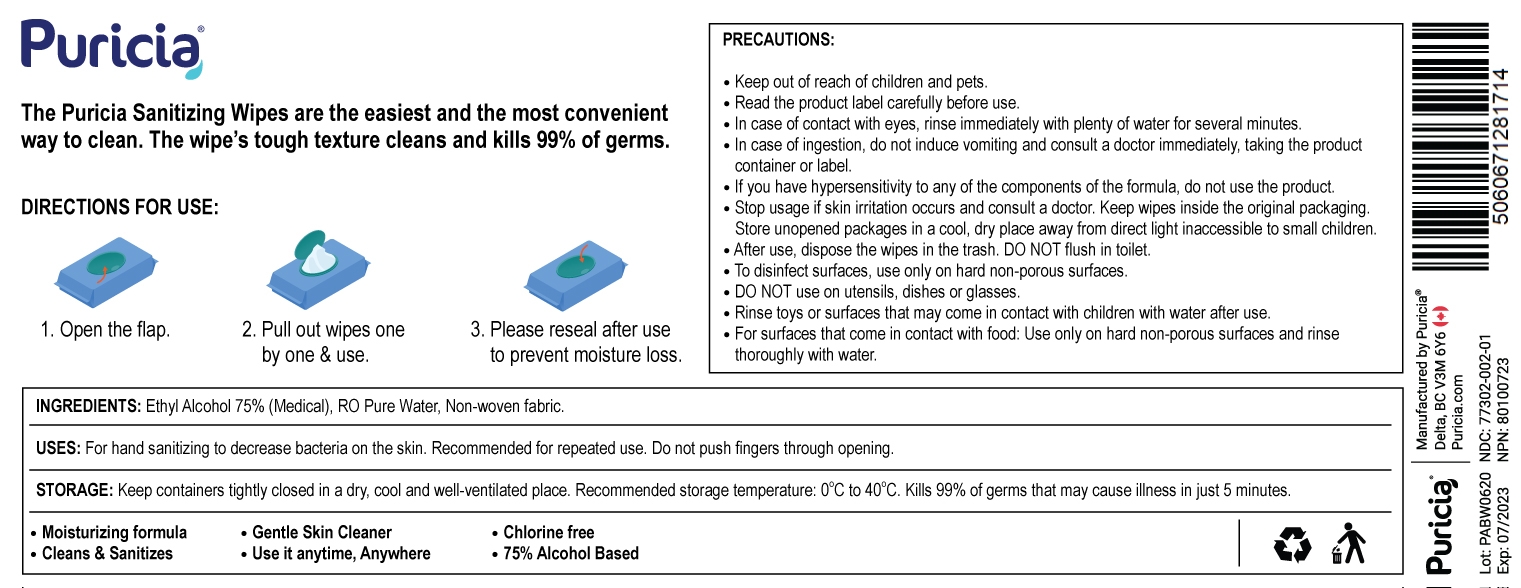
Hand Sanitizing Wipes
The Puricia Sanitizing Wipes are the easiest and the most convenient way to clean. The wipe's tough texture cleans and kills 99% of germs.
DIRECTION FOR USE:
- Open the flip.
- Pull out wipes one by one & use.
- Please reseal after use to prevent moisture loss.
USES:
For hand sanitizing to decrease bacteria on the skin. Recommended for repeated use. Do not push fingers through opening
STORAGE:
Keep containers tightly closed in a dry, cool and well-ventilated place. Recommended storage temperature: 0°C to 40°C. Kills 99% of germs that may cause illness in just 5 minutes.
- Moisturizing formula
- Cleans & Sanitizes
- Gentle Skin Cleaner
- Use it anytime, Anywhere
- Chlorine-free
- 75% Alcohol Based
PRECAUTIONS:
- keep out of reach of children and pets.
- Read the product label carefully before use.
- In case of contact with eyes, rinse immediately with plenty of water for several minutes.
- In case of ingestion, do not induce vomiting and consult a doctor immediately, taking the product container or label.
- If you have a hypersensitivity to any of the components of the formula, do not use the product.
- Stop usage if skin irritation occurs and consult a doctor. Keep wipes inside the original packaging.
- Store unopened packages in a cool, dry place away from direct light inaccessible to small children.
- After use, dispose the wipes in the trash. DO NOT flush in toilet.
- To disinfect surfaces, use only on hard non-porous surfaces.
- DO NOT use on utensils, dishes or glasses.
- Rinse toys or surfaces that may come in contact with children with water after use.
- For surfaces that come in contact with food: Use only on hard non-porous surfaces and rinse thoroughly with water.
PRECAUTIONS:
Keep out of reach of children.
Rinse toys or surfaces that may come in contact with children with water after use.
Package Label - Principal Display Panel
50 Wipes. NDC: 77302-002-01
 50 Wipes. NDC: 77302-002-01
50 Wipes. NDC: 77302-002-01
 80 Wipes. NDC: 77302-002-02
80 Wipes. NDC: 77302-002-02

80 Wipes. NDC: 77302-002-02

| PURICIA SANITIZING WIPES
ethyl alcohol 75% cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PHARMABERG INC. (204355565) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.