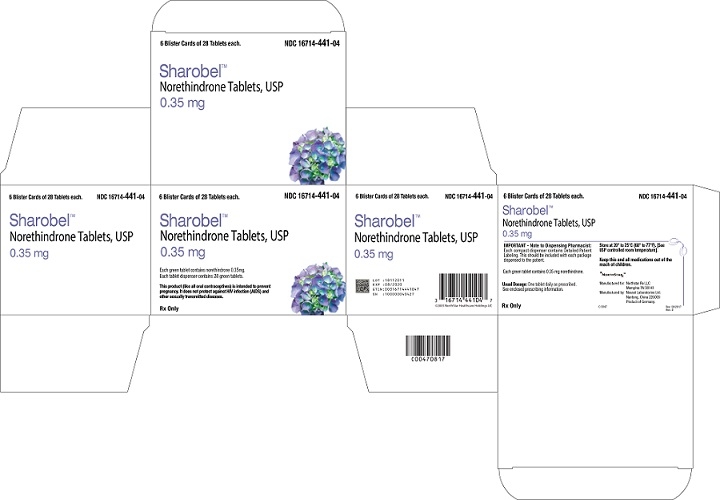
SHAROBEL- norethindrone kit
SHAROBEL by
Drug Labeling and Warnings
SHAROBEL by is a Prescription medication manufactured, distributed, or labeled by Northstar Rx LLC, Novast Laboratories, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Cigarette smoking increases the risk of serious cardiovascular side effects from oral contraceptive use. This risk increases with age and with heavy smoking (15 or more cigarettes per day) and is quite marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
-
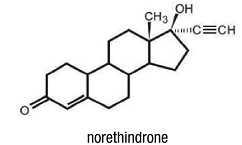
DESCRIPTION
Sharobel ™ Tablets.
Each tablet contains 0.35 mg norethindrone. Inactive ingredients include FD&C Blue No. 1 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), hydromellose, lactose monohydrate, magnesium stearate, and pregelatinized starch.
Meets USP Dissolution Test 3.
-
CLINICAL PHARMACOLOGY
1. Mode of Action
SHAROBEL ™ progestin-only oral contraceptives prevent conception by suppressing ovulation in approximately half of users, thickening the cervical mucus to inhibit sperm penetration, lowering the midcycle LH and FSH peaks, slowing the movement of the ovum through the fallopian tubes, and altering the endometrium.
2. Pharmacokinetics
Serum progestin levels peak about two hours after oral administration, followed by rapid distribution and elimination. By 24 hours after drug ingestion, serum levels are near baseline, making efficacy dependent upon rigid adherence to the dosing schedule. There are large variations in serum levels among individual users. Progestin-only administration results in lower steady-state serum progestin levels and a shorter elimination half-life than concomitant administration with estrogens.
-
INDICATIONS AND USAGE
Progestin-only oral contraceptives are indicated for the prevention of pregnancy.
2. Efficacy
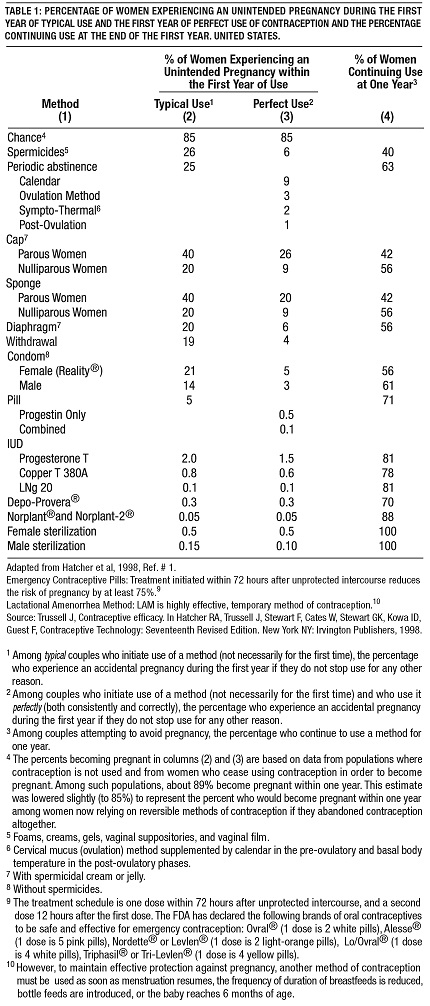
If used perfectly, the first-year failure rate for progestin-only oral contraceptives is 0.5%. However, the typical failure rate is estimated to be closer to 5%, due to late or omitted pills. Table 1 lists the pregnancy rates for users of all major methods of contraception.
SHAROBEL ™ Tablets have not been studied for and are not indicated for use in emergency contraception.
-
CONTRAINDICATIONS
Progestin-only oral contraceptives (POPs) should not be used by women who currently have the following conditions:
- Known or suspected pregnancy
- Known or suspected carcinoma of the breast
- Undiagnosed abnormal genital bleeding
- Hypersensitivity to any component of this product
- Benign or malignant liver tumors
- Acute liver disease
-
WARNINGS
Cigarette smoking increases the risk of serious cardiovascular disease. Women who use oral contraceptives should be strongly advised not to smoke.
SHAROBEL ™ does not contain estrogen and, therefore, this insert does not discuss the serious health risks that have been associated with the estrogen component of combined oral contraceptives (COCs). The healthcare professional is referred to the prescribing information of combined oral contraceptives for a discussion of those risks. The relationship between progestin-only oral contraceptives and these risks is not fully defined. The healthcare professional should remain alert to the earliest manifestation of symptoms of any serious disease and discontinue oral contraceptive therapy when appropriate.
1. Ectopic Pregnancy
The incidence of ectopic pregnancies for progestin-only oral contraceptive users is 5 per 1000 woman-years. Up to 10% of pregnancies reported in clinical studies of progestin-only oral contraceptive users are extrauterine. Although symptoms of ectopic pregnancy should be watched for, a history of ectopic pregnancy need not be considered a contraindication to use of this contraceptive method. Healthcare professionals should be alert to the possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain while on progestin-only oral contraceptives.
2. Delayed Follicular Atresia/Ovarian Cysts
If follicular development occurs, atresia of the follicle is sometimes delayed and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally these enlarged follicles disappear spontaneously. Often they are asymptomatic; in some cases they are associated with mild abdominal pain. Rarely they may twist or rupture, requiring surgical intervention.
3. Irregular Genital Bleeding
Irregular menstrual patterns are common among women using progestin-only oral contraceptives. If genital bleeding is suggestive of infection, malignancy or other abnormal conditions, such nonpharmacologic causes should be ruled out. If prolonged amenorrhea occurs, the possibility of pregnancy should be evaluated.
4. Carcinoma of the Breast and Reproductive Organs
Some epidemiological studies of oral contraceptive users have reported an increased relative risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. These studies have predominantly involved combined oral contraceptives and there is insufficient data to determine whether the use of POPs similarly increases the risk.
A meta-analysis of 54 studies found a small increase in the frequency of having breast cancer diagnosed for women who were currently using combined oral contraceptives or had used them within the past ten years.
This increase in the frequency of breast cancer diagnosis, within ten years of stopping use, was generally accounted for by cancers localized to the breast. There was no increase in the frequency of having breast cancer diagnosed ten or more years after cessation of use.
Women with breast cancer should not use oral contraceptives because the role of female hormones in breast cancer has not been fully determined.
Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. There is insufficient data to determine whether the use of POPs increases the risk of developing cervical intraepithelial neoplasia.
5. Hepatic Neoplasia
Benign hepatic adenomas are associated with combined oral contraceptive use, although the incidence of benign tumors is rare in the United States. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in combined oral contraceptive users. However, these cancers are rare in the U.S. There is insufficient data to determine whether POPs increase the risk of developing hepatic neoplasia.
-
PRECAUTIONS
1. General
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
2. Physical Examination and Follow-up
It is considered good medical practice for sexually active women using oral contraceptives to have annual history and physical examinations. The physical examination may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the healthcare professional.
3. Carbohydrate and Lipid Metabolism
Some users may experience slight deterioration in glucose tolerance, with increases in plasma insulin but women with diabetes mellitus who use progestin-only oral contraceptives do not generally experience changes in their insulin requirements. Nonetheless, prediabetic and diabetic women in particular should be carefully monitored while taking POPs.
Lipid metabolism is occasionally affected in that HDL, HDL2, and apolipoprotein A-I and A-II may be decreased; hepatic lipase may be increased. There is usually no effect on total cholesterol, HDL3, LDL, or VLDL.
4. Drug Interactions
The effectiveness of progestin-only pills is reduced by hepatic enzyme-inducing drugs such as the anticonvulsants phenytoin, carbamazepine, and barbiturates, and the antituberculosis drug rifampin. No significant interaction has been found with broad-spectrum antibiotics.
Herbal products containing St. John’s Wort (Hypericum perforatum) may induce hepatic enzymes (cytochrome P450) and p-glycoprotein transporter and may reduce the effectiveness of contraceptive steroids. This may also result in breakthrough bleeding.
Concurrent use of bosentan and norethindrone containing products may result in decreased concentrations of these contraceptive hormones thereby increasing the risk of unintended pregnancy and unscheduled bleeding.
5. Interactions with Laboratory Tests
The following endocrine tests may be affected by progestin-only oral contraceptive use:
- Sex hormone-binding globulin (SHBG) concentrations may be decreased.
- Thyroxine concentrations may be decreased, due to a decrease in thyroid binding globulin (TBG).
7. Pregnancy
Many studies have found no effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted have not demonstrated significant adverse effects. It is nonetheless prudent to rule out suspected pregnancy before initiating any hormonal contraceptive use.
8. Nursing Mothers
In general, no adverse effects have been found on breastfeeding performance or on the health, growth or development of the infant. However, isolated post-marketing cases of decreased milk production have been reported. Small amounts of progestins pass into the breast milk of nursing mothers, resulting in detectable steroid levels in infant plasma.
9. Pediatric Use
Safety and efficacy of Norethindrone 0.35mg Tablets have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
10. Fertility Following Discontinuation
The limited available data indicate a rapid return of normal ovulation and fertility following discontinuation of progestin-only oral contraceptives.
11. Headache
The onset or exacerbation of migraine or development of severe headache with focal neurological symptoms which is recurrent or persistent requires discontinuation of progestin-only contraceptives and evaluation of the cause.
INFORMATION FOR THE PATIENT
1. See "Detailed Patient Labeling" for detailed information.
2. Counseling issues
The following points should be discussed with prospective users before prescribing progestin-only oral contraceptives:
- The necessity of taking pills at the same time every day, including throughout all bleeding episodes.
- The need to use a backup method such as condoms and spermicide for the next 48 hours whenever a progestin-only oral contraceptive is taken 3 or more hours late.
- The potential side effects of progestin-only oral contraceptives, particularly menstrual irregularities.
- The need to inform the healthcare professional of prolonged episodes of bleeding, amenorrhea or severe abdominal pain.
- The importance of using a barrier method in addition to progestin-only oral contraceptives if a woman is at risk of contracting or transmitting STDs/HIV.
-
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Northstar Rx LLC. Toll-Free at 1-800-206-7821 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Adverse reactions reported with the use of POPs include:
- Menstrual irregularity is the most frequently reported side effect.
- Frequent and irregular bleeding are common, while long duration of bleeding episodes and amenorrhea are less likely.
- Headache, breast tenderness, nausea, and dizziness are increased among progestin-only oral contraceptive users in some studies.
- Androgenic side effects such as acne, hirsutism, and weight gain occur rarely.
The following adverse reactions were also reported in clinical trials or during post-marketing experience: Gastrointestinal Disorders: vomiting, abdominal pain; General Disorders and Administration Site Conditions: fatigue, edema; Psychiatric Disorders: depression, nervousness; Musculoskeletal and Connective Tissue Disorders: pain in extremity; Reproductive System and Breast Disorders: genital discharge; breast pain, menstruation delayed, suppressed lactation, vaginal hemorrhage, menorrhagia, withdrawal bleed when product is stopped; Immune System Disorders: anaphylactic/anaphylactoid reaction, hypersensitivity; Hepatobiliary Disorders: hepatitis, jaundice cholestatic; Skin and Subcutaneous Tissue Disorders: alopecia, rash, rash pruritic.
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
SHAROBEL™ (0.35 mg Norethindrone Tablets, USP) is available in a compact card (NDC: 16714-441-01) containing 28 green, biconvex, round tablets imprinted "V2" on one side.
SHAROBEL™ is available in the following configurations:
Carton of 1 NDC: 16714-441-02
Carton of 3 NDC: 16714-441-03
Carton of 6 NDC: 16714-441-04Store at 20° to 25°C (68° to 77°F). [See USP for Controlled Room Temperature.]
Keep out this and all medication of the reach of children.
REFERENCE
1. McCann M, and Potter L. Progestin-Only Oral Contraceptives: A Comprehensive Review. Contraception, 50:60 (Suppl. 1), December 1994.
2. Van Giersbergen PLM, Halabi A, Dingemanse J. Pharmacokinetic interaction between bosentan and the oral contraceptives norethisterone and ethinyl estradiol. Int J Clin Pharmacol Ther 2006;44(3):113-118.
3. Truitt ST, Fraser A, Gallo ME, Lopez LM, Grimes DA and Schulz KF. Combined hormonal versus nonhormonal versus progestin-only contraception in lactation (Review). The Cochrane Collaboration. 2007, Issue 3.
4. Halderman, LD and Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol.; 186 (6): 1250-1258.
5. Ostrea EM, Mantaring III JB, Silvestre MA. Drugs that affect the fetus and newborn infant via the placenta or breast milk. Pediatr Clin N Am; 51(2004): 539-579.
6. Cooke ID, Back DJ, Shroff NE: Norethisterone concentration in breast milk and infant and maternal plasma during ethynodiol diactetate administration. Contraception 1985; 31:611-21.
7. 2008 USPC Official:12/1/08-4/30/09, USP Monographs: Norethindrone Tablets (page 1 of 5).
-
PATIENT PACKAGE INSERT
SHAROBEL™ (Norethindrone Tablets, USP)
This product (like all oral contraceptives) is used to prevent pregnancy. It does not protect against HIV infection (AIDS) or other sexually transmitted diseases.
DESCRIPTION
SHAROBEL™ Tablets
Each tablet contains 0.35 mg norethindrone. Inactive ingredients include FD&C Blue No. 1 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), hydromellose, lactose monohydrate, magnesium stearate, and pregelatinized starch.
INTRODUCTION
This leaflet is about birth control pills that contain one hormone, a progestin. Please read this leaflet before you begin to take your pills. It is meant to be used along with talking with your healthcare professional.
Progestin-only pills are often called "POPs" or "the minipill." POPs have less progestin than the combined birth control pill (or "the pill") which contains both an estrogen and a progestin.
HOW EFFECTIVE ARE POPs?
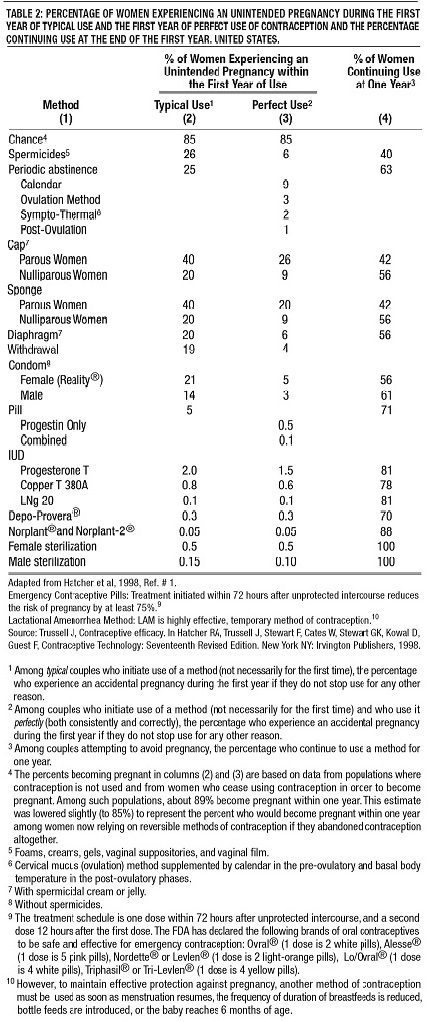
About 1 in 200 POP users will get pregnant in the first year if they all take POPs perfectly (that is, on time, every day). About 1 in 20 "typical" POP users (including women who are late taking pills or miss pills) gets pregnant in the first year of use. Table 2 will help you compare the efficacy of different methods.
SHAROBEL™ Tablets have not been studied for and are not indicated for use in emergency contraception.
HOW DO POPs WORK?
POPs can prevent pregnancy in different ways including:
- They make the cervical mucus at the entrance to the womb (the uterus) too thick for the sperm to get through to the egg.
- They prevent ovulation (release of the egg from the ovary) in about half of the cycles.
- They also affect other hormones, the fallopian tubes and the lining of the uterus.
- If there is any chance you may be pregnant.
- If you have breast cancer.
- If you have bleeding between your periods that has not been diagnosed.
- If you are taking certain drugs for epilepsy (seizures) or for TB, or medicine for pulmonary hypertension or certain herbal products. (See "Using POPs with Other Medicines" below.)
- If you are hypersensitive, or allergic, to any component of this product.
- If you have liver tumors, either benign or cancerous.
- If you have acute liver disease.
Cigarette smoking greatly increases the possibility of suffering heart attacks and strokes. Women who use oral contraceptives are strongly advised not to smoke.
WARNING:
If you have sudden or severe pain in your lower abdomen or stomach area, you may have an ectopic pregnancy or an ovarian cyst. If this happens, you should contact your healthcare professional immediately.
Ectopic Pregnancy
An ectopic pregnancy is a pregnancy outside the womb. Because POPs protect against pregnancy, the chance of having a pregnancy outside the womb is very low. If you do get pregnant while taking POPs, you have a slightly higher chance that the pregnancy will be ectopic than do users of some other birth control methods.
Ovarian Cysts
These cysts are small sacs of fluid in the ovary. They are more common among POP users than among users of most other birth control methods. They usually disappear without treatment and rarely cause problems.
Cancer of the Reproductive Organs and Breasts
Some studies in women who use combined oral contraceptives that contain both estrogen and a progestin have reported an increase in the risk of developing breast cancer, particularly at a younger age and apparently related to duration of use. There is insufficient data to determine whether the use of POPs similarly increases this risk.
A meta-analysis of 54 studies found a small increase in the frequency of having breast cancer diagnosed for women who were currently using combined oral contraceptives or had used them within the past ten years. This increase in the frequency of breast cancer diagnosis, within ten years of stopping use, was generally accounted for by cancers localized to the breast. There was no increase in the frequency of having breast cancer diagnosed ten or more years after cessation of use.
Some studies have found an increase in the incidence of cancer of the cervix in women who use oral contraceptives. However, this finding may be related to factors other than the use of oral contraceptives and there is insufficient data to determine whether the use of POPs increases the risk of developing cancer of the cervix.
Liver Tumors
In rare cases, combined oral contraceptives can cause benign but dangerous liver tumors. These benign liver tumors can rupture and cause fatal internal bleeding. In addition, some studies report an increased risk of developing liver cancer among women who use combined oral contraceptives. However, liver cancers are rare. There is insufficient data to determine whether POPs increase the risk of liver tumors.
Diabetic Women
Diabetic women taking POPs do not generally require changes in the amount of insulin they are taking. However, your healthcare professional may monitor you more closely under these conditions.
SEXUALLY TRANSMITTED DISEASES (STDs)
WARNING: POPs do not protect against getting or giving someone HIV (AIDS) or any other STD, such as chlamydia, gonorrhea, genital warts or herpes.
SIDE EFFECTS
Irregular Bleeding:
The most common side effect of POPs is a change in menstrual bleeding. Your periods may be either early or late, and you may have some spotting between periods. Taking pills late or missing pills can result in some spotting or bleeding.
Other Side Effects:
Less common side effects include headaches, tender breasts, nausea, vomiting, dizziness, and fatigue. Depression, nervousness, leg pain, vaginal discharge, fluid retention, allergic reactions, jaundice or a yellowing of the skin or eyeballs, loss of scalp hair, rash/itchy rash, weight gain, acne and extra hair on your face and body have been reported, but are rare.
If you are concerned about any of these side effects, check with your healthcare professional.
USING POPs WITH OTHER MEDICINES
Before taking a POP, inform your healthcare professional of any other medication, including over-the-counter medicine, that you may be taking.
These medicines can make POPs less effective:
Medicines for seizures such as:
● Phenytoin (Dilantin®)
● Carbamazepine (Tegretol)
● Phenobarbital
Medicine for TB:
● Rifampin (Rifampicin)
Medicine for pulmonary hypertension such as:
● Bosentan (Tracleer®)
Herbal products such as:
● St. John’s Wort
Before you begin taking any new medicines be sure your healthcare professional knows you are taking a progestin-only birth control pill.
IMPORTANT POINTS TO REMEMBER
- POPs must be taken at the same time every day, so choose a time and then take the pill at that same time every day. Every time you take a pill late, and especially if you miss a pill, you are more likely to get pregnant.
- Start the next pack the day after the last pack is finished. There is no break between packs. Always have your next pack of pills ready.
- You may have some menstrual spotting between periods. Do not stop taking your pills if this happens.
- If you vomit soon after taking a pill, use a backup method (such as a condom and/or a spermicide) for 48 hours.
- If you want to stop taking POPs, you can do so at any time, but, if you remain sexually active and don’t wish to become pregnant, be certain to use another birth control method.
- If you are not sure about how to take POPs, ask your healthcare professional.
STARTING POPs
- It’s best to take your first POP on the first day of your menstrual period.
- If you decide to take your first POP on another day, use a backup method (such as a condom and/or a spermicide) every time you have sex during the next 48 hours.
- If you have had a miscarriage or an abortion, you can start POPs the next day.
IF YOU ARE LATE OR MISS TAKING YOUR POPs
● If you are more than 3 hours late or you miss one or more POPs:
1. TAKE a missed pill as soon as you remember that you missed it,
2. THEN go back to taking POPs at your regular time,
3. BUT be sure to use a backup method (such as a condom and/or a spermicide) every time you have sex for the next 48 hours.
● If you are not sure what to do about the pills you have missed, keep taking POPs and use a backup method until you can talk to your healthcare professional.
IF YOU ARE BREASTFEEDING
- If you are fully breastfeeding (not giving your baby any food or formula), you may start your pills 6 weeks after delivery.
- If you are partially breastfeeding (giving your baby some food or formula), you should start taking pills by 3 weeks after delivery.
IF YOU ARE SWITCHING PILLS
- If you are switching from the combined pills to POPs, take the first POP the day after you finish the last active combined pill. Do not take any of the 7 inactive pills from the combined pill pack. You should know that many women have irregular periods after switching to POPs, but this is normal and to be expected.
- If you are switching from POPs to the combined pills, take the first active combined pill on the first day of your period, even if your POPs pack is not finished.
- If you switch to another brand of POPs, start the new brand anytime.
- If you are breastfeeding, you can switch to another method of birth control at any time, except do not switch to the combined pills until you stop breastfeeding or at least until 6 months after delivery.
If you think you are pregnant, contact your healthcare professional. Even though research has shown that POPs do not cause harm to the unborn baby, it is always best not to take any drugs or medicines that you don’t need when you are pregnant.
You should get a pregnancy test:
- If your period is late and you took one or more pills late or missed taking them and had sex without a backup method.
- Anytime it has been more than 45 days since the beginning of your last period.
WILL POPs AFFECT YOUR ABILITY TO GET PREGNANT LATER?
If you want to become pregnant, simply stop taking POPs. POPs will not delay your ability to get pregnant.
BREASTFEEDING
If you are breastfeeding, POPs will not affect the quality or amount of your breast milk or the health of your nursing baby. However, isolated cases of decreased milk production have been reported.
OVERDOSE
No serious problems have been reported when many pills were taken by accident, even by a small child, so there is usually no reason to treat an overdose.
OTHER QUESTIONS OR CONCERNS
If you have any questions or concerns, check with your healthcare professional. You can also ask for the more detailed "Professional Labeling" written for doctors and other healthcare professionals.
HOW TO STORE YOUR POPs
Store at 20° to 25°C (68° to 77°F). [See USP for Controlled Room Temperature.]
Rx Only
Keep out this and all medication of the reach of children.
Northstar Rx LLC
Memphis, TN 38141
Manufactured by:
Novast Laboratories Ltd.
Nantong, China 226009
I0066 Iss.11/2015 Rev.A
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHAROBEL
norethindrone kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16714-441 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16714-441-01 1 in 1 PACKET 09/13/2013 1 1 in 1 BLISTER PACK 2 NDC: 16714-441-02 1 in 1 CARTON 01/01/2020 2 1 in 1 BLISTER PACK 3 NDC: 16714-441-03 3 in 1 CARTON 01/01/2020 3 1 in 1 BLISTER PACK 4 NDC: 16714-441-04 6 in 1 CARTON 09/13/2013 4 1 in 1 BLISTER PACK Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 28 Part 1 of 1 SHAROBEL
norethindrone tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NORETHINDRONE (UNII: T18F433X4S) (NORETHINDRONE - UNII:T18F433X4S) NORETHINDRONE 0.35 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color GREEN Score no score Shape ROUND Size 5mm Flavor Imprint Code V2 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200961 09/13/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200961 09/13/2013 Labeler - Northstar Rx LLC (830546433) Registrant - Novast Laboratories, Ltd. (527695995) Establishment Name Address ID/FEI Business Operations Novast Laboratories, Ltd. 527695995 MANUFACTURE(16714-441)
Trademark Results [SHAROBEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SHAROBEL 85927868 4676037 Live/Registered |
Novast Laboratories, Inc. 2013-05-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.