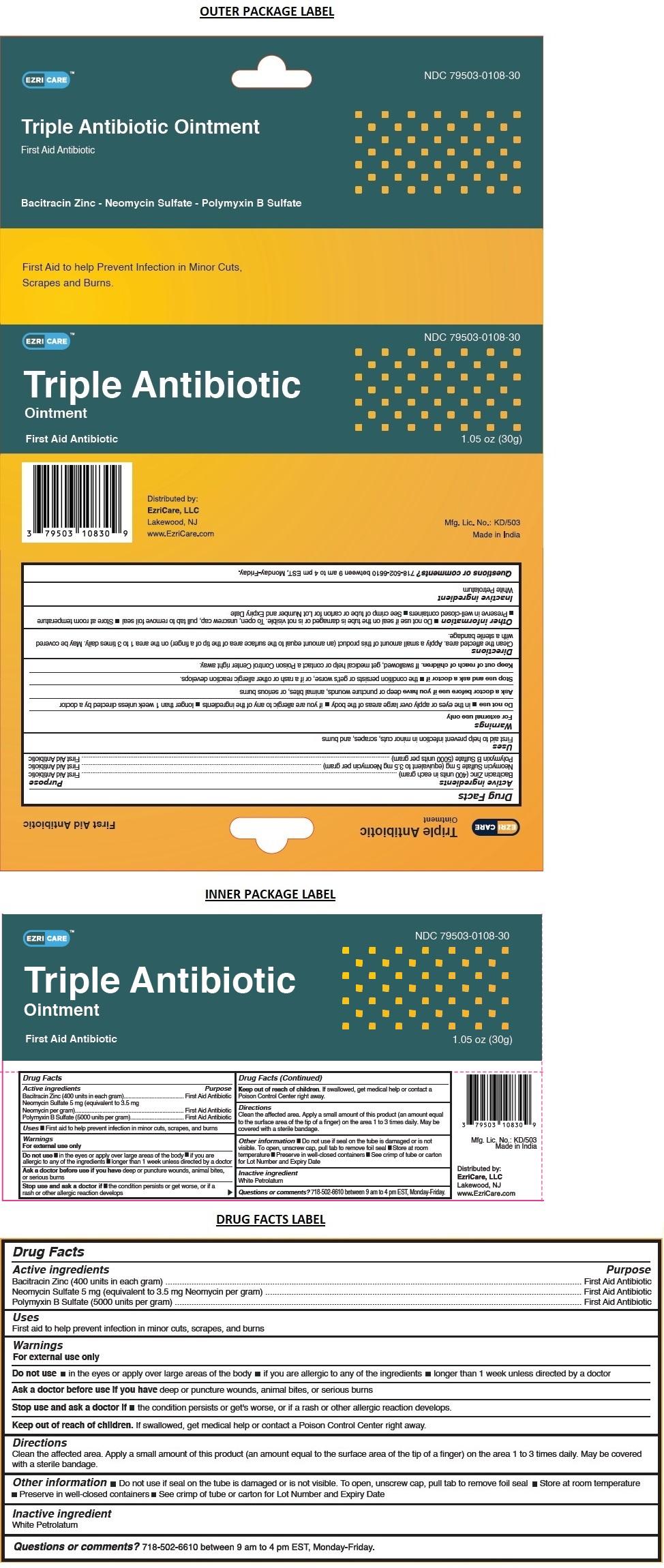
EZRI CARETM Triple Antibiotic Ointment
Triple Antibiotic by
Drug Labeling and Warnings
Triple Antibiotic by is a Otc medication manufactured, distributed, or labeled by Ezricare Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment
Ezricare Llc
----------
EZRI CARETM Triple Antibiotic Ointment
Active ingredients
Bacitracin Zinc (400 units in each gram)
Neomycin Sulfate 5 mg (equivalent to 3.5 mg Neomycin per gram)
Polymyxin B Sulfate (5000 units per gram)
Warnings
For external use only
Do not use in the eyes or apply over large areas of the body if you are allergic to any of the ingredients longer than 1 week unless directed by a doctor
Ask a doctor before use if you have deep or puncture wounds, animal bites, or serious burns
Stop use and ask a doctor if the condition persists or get's worse, or if a rash or other allergic reaction develops
Directions
Clean the affected area. Apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily. May be covered with a sterile bandage.
| TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ezricare Llc (117573818) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.