Biotipo Pharma Medical Biogel
Biotipo Pharma Medical Biogel by
Drug Labeling and Warnings
Biotipo Pharma Medical Biogel by is a Otc medication manufactured, distributed, or labeled by Unibeleza Industria e Comercio de Cosmeticos Ltda. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
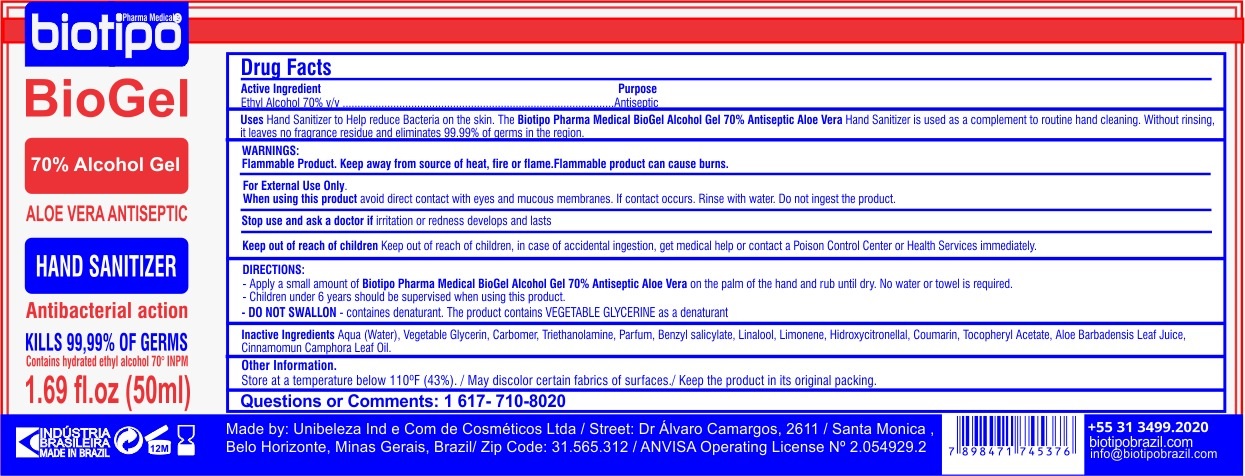
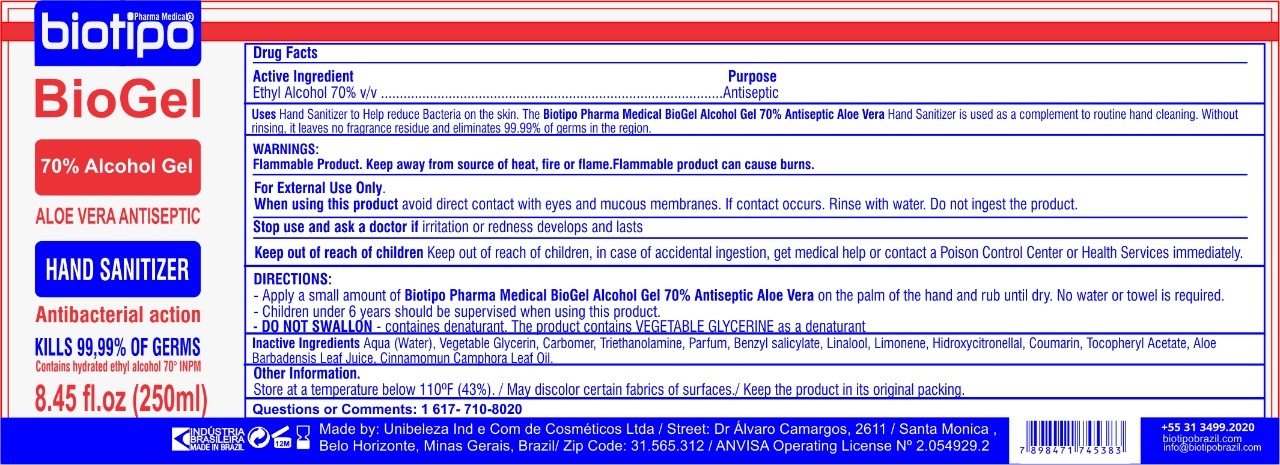
BIOTIPO PHARMA MEDICAL BIOGEL- alcohol gel
Unibeleza Industria e Comercio de Cosmeticos Ltda
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Biotipo Pharma Medical Biogel
Uses
Hand Sanitizer to Help reduce Bacteria on the skin. The Biotipo Pharma Medical BioGel Alcohol Gel 70% Antiseptic Aloe Vera Hand Sanitizer is used as a complement to routine hand cleaning. Without rinising, it leaves no fragrance residue and eliminates 99.99% of germs in the region.
WARNINGS:
Flammable Product. Keep away from source of heat, fire or flame. FLammable product can cause burns.
For External Use Only.
DIRECTIONS:
-Apply a small amount of Biotipo Pharma Medical BioGel Alcohol Gel 70% Antiseptic Aloe Vera on the palm of the hand and rub until dry. No water or towel is required.
-Children under 6 years should be supervised when using this product.
- DO NOT SWALLON - containes denaturant. THe product contains VEGETABLE GLYCERINE as a denaturant
Inactive Ingredients
Aqua(Water), Vegetable Glycerin, Carbomer, Triethanolamine, Parfum, Benzyl Salicylate, Linalool, Limonene, Hidroxycitronellal, Coumarin, Tocopheryl Acetate, Aloe Babarbadensis Leaf Juice, Cinnamomun Camphora Leaf oil.
| BIOTIPO PHARMA MEDICAL BIOGEL
alcohol gel |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Unibeleza Industria e Comercio de Cosmeticos Ltda (679072947) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.