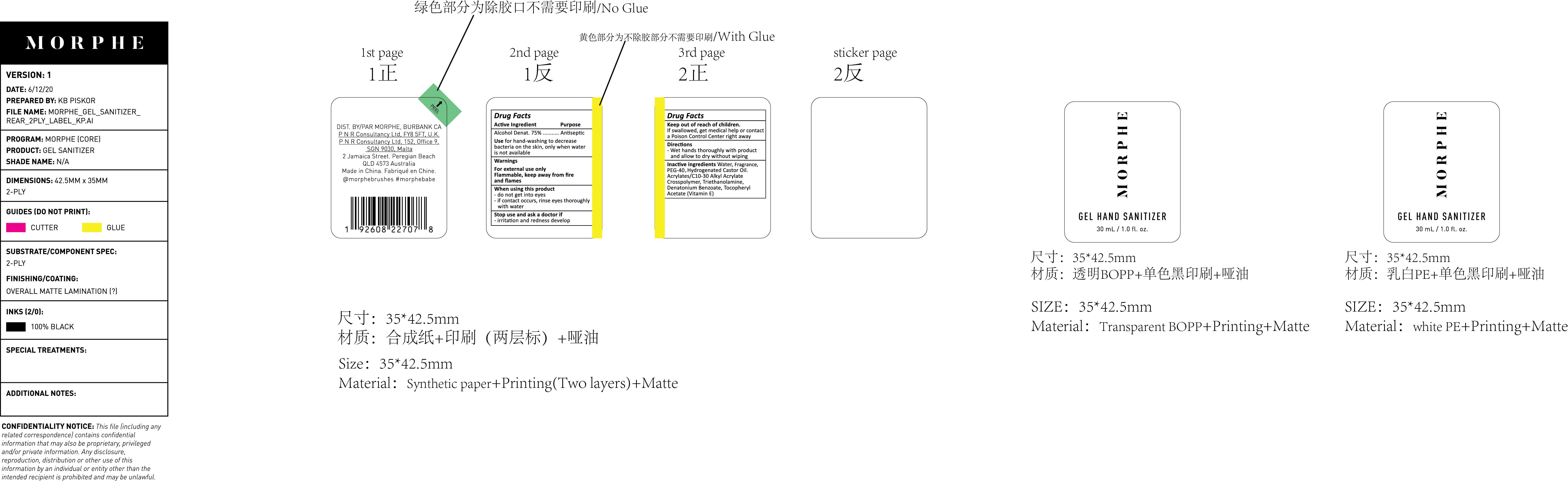
AHS-001 clear, cucumber BBC
Morphe gel hand sanitizer by
Drug Labeling and Warnings
Morphe gel hand sanitizer by is a Otc medication manufactured, distributed, or labeled by BBC GROUP LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MORPHE GEL HAND SANITIZER 01- alochol gel
BBC GROUP LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AHS-001 clear, cucumber
BBC
When using this product
- do not get into eyes.
-if contact occurs, rinse eyes thoroughly with water.
| MORPHE GEL HAND SANITIZER
01
alochol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - BBC GROUP LIMITED (421270060) |
Revised: 7/2020
Document Id: aa87863d-ba85-f55e-e053-2995a90aa3b0
Set id: a9d6bc2d-9929-55df-e053-2995a90abaa0
Version: 3
Effective Time: 20200716
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.