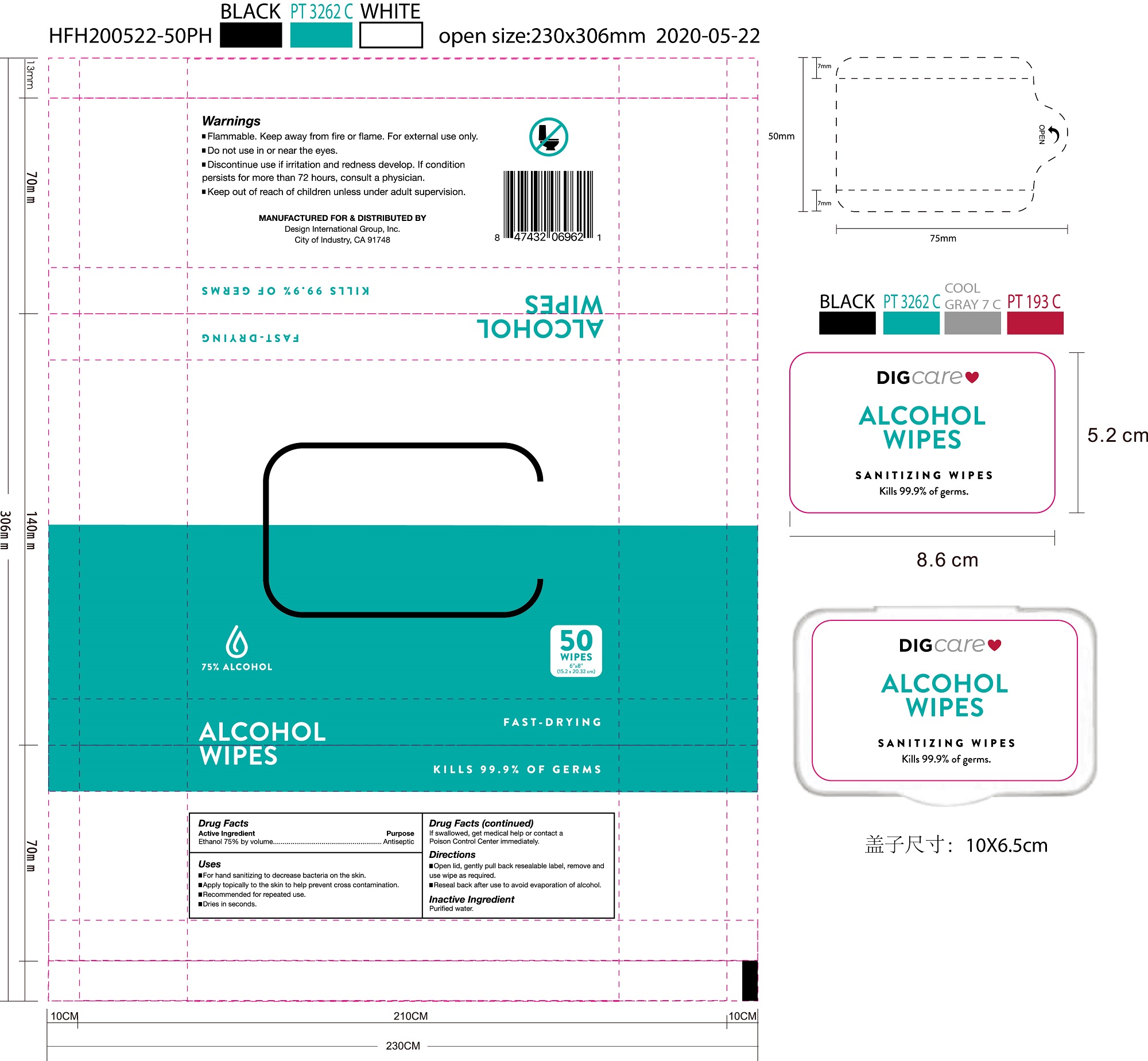
75109-512 75% alcohol Digcare Alcohol Wipes
Digcare Alcohol Wipes by
Drug Labeling and Warnings
Digcare Alcohol Wipes by is a Otc medication manufactured, distributed, or labeled by Kangna (Zhejiang) Medical Supplies Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIGCARE ALCOHOL WIPES- alcohol cloth
Kangna (Zhejiang) Medical Supplies Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
75109-512 75% alcohol Digcare Alcohol Wipes
USE
for hand sanitizing to decrease bacteria on the skin
apply topically to the skin to help prevent cross contamination
recommended for repeated use
dries in seconds
Warning
Flammable. Keep away from fire or flame. For external use only.
Do not use in or near the eyes.
Discontinue use if irritation and redness develop. If condition
persists for more than 72 hours, consult a physician.
| DIGCARE ALCOHOL WIPES
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kangna (Zhejiang) Medical Supplies Co., Ltd. (554530173) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kangna (Zhejiang) Medical Supplies Co., Ltd. | 554530173 | manufacture(75109-512) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.