Ear Pain MD (E007) by Eosera Inc Ear Pain MD (E007)
Ear Pain MD (E007) by
Drug Labeling and Warnings
Ear Pain MD (E007) by is a Otc medication manufactured, distributed, or labeled by Eosera Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EAR PAIN MD (E007)- ear pain md liquidÂ
Eosera Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
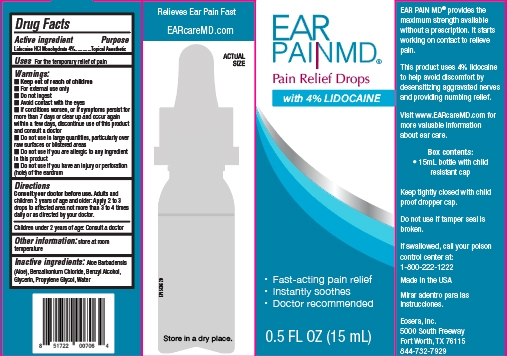
Ear Pain MD (E007)
Warnings
For external use only, Do not ingest, Avoid contact with the eyes, If conditions worsen, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor, Do not use in large quantities, particularly over raw surfaces or blisterred areas, Do not use if you are allergic to any ingredient in this product, Do not use if you have an injury or perforation (hole) of the eardrum
Directions
Consult your doctor before use. Adults and children 2 years of age and older: Apply 2 to 3 drops to affected area not more than 3 to 4 times daily or as directed by your doctor.
Children under 2 years of age: Consult a doctor
| EAR PAIN MD (E007)Â
ear pain md liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler -Â Eosera Inc (079789050) |
| Registrant -Â Eosera Inc (079789050) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 See image
See image