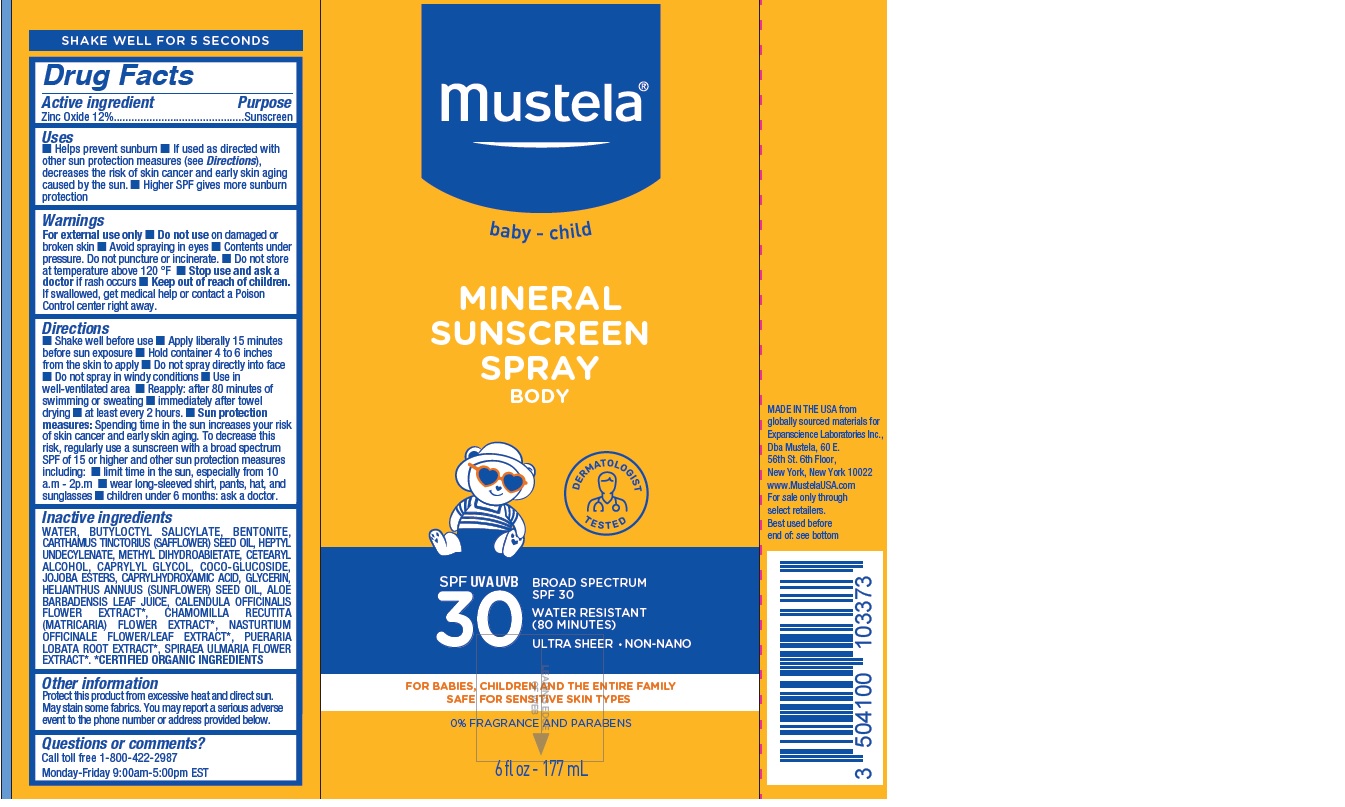
MUSTELA MINERAL SUNSCREEN SPRAY BROAD SPECTRUM SPF 30
MUSTELA MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 by
Drug Labeling and Warnings
MUSTELA MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 by is a Otc medication manufactured, distributed, or labeled by Expanscience Laboratories d/b/a Mustela. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MUSTELA MINERAL SUNSCREEN BROAD SPECTRUM SPF 30- zinc oxide spray
Expanscience Laboratories d/b/a Mustela
----------
MUSTELA MINERAL SUNSCREEN SPRAY BROAD SPECTRUM SPF 30
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Higher SPF gives more sunburn protection
Warnings
For external use only
Directions
- Shake well before use
- Apply liberally 15 minutes before sun exposure
- Hold container 4 to 6 inches from the skin to apply
- Do not spray directly into face
- Do not spray in windy conditions
- Use in well-ventilated area
- Reapply: after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours.
- Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m - 2p.m
- wear long-sleeved shirt, pants, hat, and sunglasses
- children under 6 months: ask a doctor.
Inactive ingredients
WATER, BUTYLOCTYL SALICYLATE, BENTONITE, CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, HEPTYL UNDECYLENATE, METHYL DIHYDROABIETATE, CETEARYL ALCOHOL, CAPRYLYL GLYCOL, COCO-GLUCOSIDE,
JOJOBA ESTERS, CAPRYLHYDROXAMIC ACID, GLYCERIN, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, ALOE BARBADENSIS LEAF JUICE, CALENDULA OFFICINALIS FLOWER EXTRACT*, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT*, NASTURTIUM OFFICINALE FLOWER/LEAF EXTRACT*, PUERARIA LOBATA ROOT EXTRACT*, SPIRAEA ULMARIA FLOWER EXTRACT*. *CERTIFIED ORGANIC INGREDIENTS
Other information
Protect this product from excessive heat and direct sun. May stain some fabrics. You may report a serious adverse event to the phone number or address provided below.
| MUSTELA MINERAL SUNSCREEN BROAD SPECTRUM SPF 30
zinc oxide spray |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Expanscience Laboratories d/b/a Mustela (181191057) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.