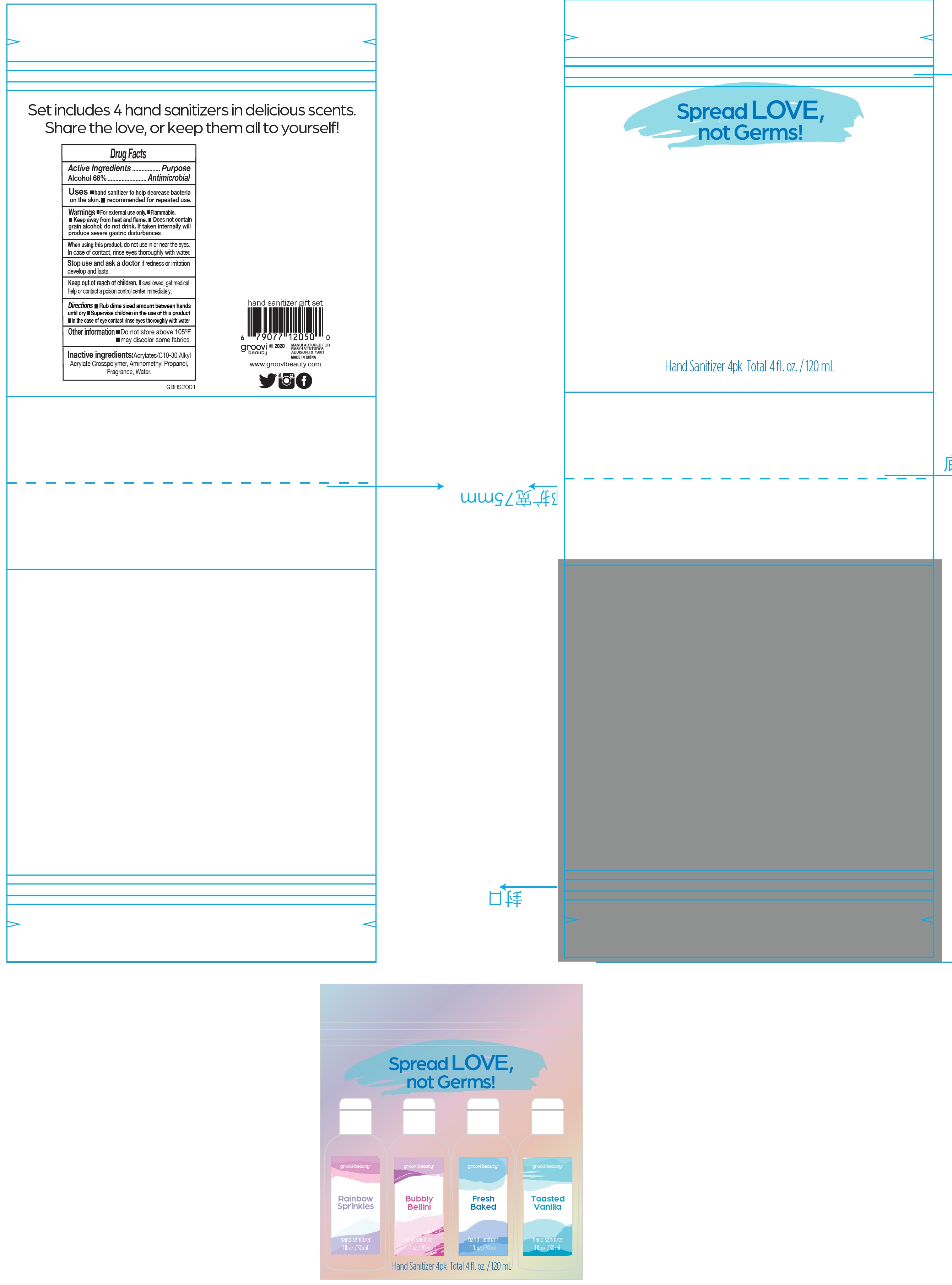
SPREAD LOVE NOT GERMS RAINBOW SPRINKLES HAND SANITIZER
alcohol kit |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 62651-039 |
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 62651-039-00 | 1 in 1 KIT | 10/15/2020 | 10/15/2021 |
|
| Quantity of Parts |
| Part # | Package Quantity | Total Product Quantity |
| Part 1 | 1 BOTTLE | 29 mL |
| Part 2 | 1 BOTTLE | 29 mL |
| Part 3 | 1 BOTTLE | 29 mL |
| Part 4 | 1 BOTTLE | 29 mL |
|
| Part 1 of 4 |
HAND SANITIZER TOASTED VANILLA
alcohol gel |
|
| Product Information |
| Item Code (Source) | NDC: 62651-032 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 0.66 mL in 1 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) | |
| AMINOMETHYLPROPANOL (UNII: LU49E6626Q) | |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 62651-032-01 | 29 mL in 1 BOTTLE; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 10/15/2020 | 10/15/2021 |
|
| Part 2 of 4 |
HAND SANITIZER FRESH BAKED
alcohol gel |
|
| Product Information |
| Item Code (Source) | NDC: 62651-033 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 0.66 mL in 1 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) | |
| AMINOMETHYLPROPANOL (UNII: LU49E6626Q) | |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 62651-033-01 | 29 mL in 1 BOTTLE; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 10/15/2020 | 10/15/2021 |
|
| Part 3 of 4 |
HAND SANITIZER BUBBLY BELLINI
alcohol gel |
|
| Product Information |
| Item Code (Source) | NDC: 62651-034 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 0.66 mL in 1 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) | |
| AMINOMETHYLPROPANOL (UNII: LU49E6626Q) | |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 62651-034-01 | 29 mL in 1 BOTTLE; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 10/15/2020 | 10/15/2021 |
|
| Part 4 of 4 |
HAND SANITIZER RAINBOW SPRINKLES
alcohol gel |
|
| Product Information |
| Item Code (Source) | NDC: 62651-035 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 0.66 mL in 1 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) | |
| AMINOMETHYLPROPANOL (UNII: LU49E6626Q) | |
| WATER (UNII: 059QF0KO0R) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 62651-035-01 | 29 mL in 1 BOTTLE; Type 0: Not a Combination Product | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 10/15/2020 | 10/15/2021 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 10/15/2020 | 10/15/2021 |
|