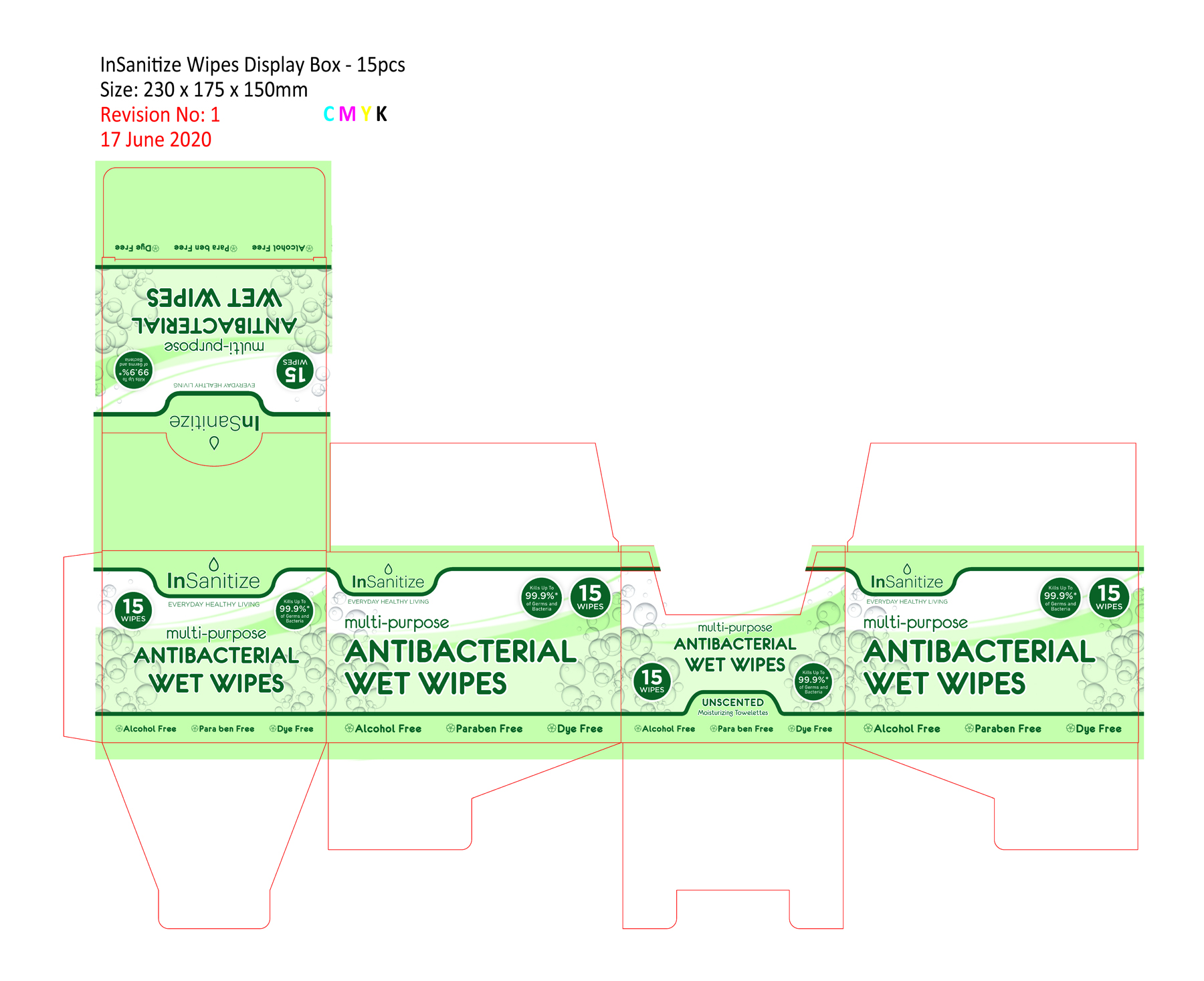
Multi-purpose Antibacterial Wet Wipes by Supreme Premium Products,Inc.
Multi-purpose Antibacterial Wet Wipes by
Drug Labeling and Warnings
Multi-purpose Antibacterial Wet Wipes by is a Otc medication manufactured, distributed, or labeled by Supreme Premium Products,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MULTI-PURPOSE ANTIBACTERIAL WET WIPES- benzalkonium chloride cloth
Supreme Premium Products,Inc.
----------
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Open using a label attached, pull out the wipe, and then seal the opening by sticking back the label to prevent the wipe from drying out.
Other information
The expiry date is stated on the packaging, Store at -20℃ to +30℃,away from sunlight.
| MULTI-PURPOSE ANTIBACTERIAL WET WIPES
benzalkonium chloride cloth |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Supreme Premium Products,Inc. (610328981) |
Revised: 12/2025
Document Id: 472862de-ca58-ecd0-e063-6394a90afd26
Set id: aa1e94f8-d808-1ad7-e053-2a95a90a3f94
Version: 2
Effective Time: 20251230