Alcohol Wipe by YAHON ENTERPRISE CO.,LTD Yahon, 001-01
Alcohol Wipe by
Drug Labeling and Warnings
Alcohol Wipe by is a Otc medication manufactured, distributed, or labeled by YAHON ENTERPRISE CO.,LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALCOHOL WIPE- alcohol cloth
YAHON ENTERPRISE CO.,LTD
----------
Yahon, 001-01
Ask a doctor before use if you have deep or puncture wounds, animal bites serious burns
Avoid contact with eyes. If in eyes, remove contact lenses (if any), rinse slowly with water or get medical help.
| ALCOHOL WIPE
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - YAHON ENTERPRISE CO.,LTD (555347945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| YAHON ENTERPRISE CO.,LTD | 555347945 | manufacture(79618-001) | |
Revised: 10/2024
Document Id: 236fb98b-b1cc-82cf-e063-6394a90ab8ed
Set id: aa216297-007a-13f4-e053-2a95a90aaf23
Version: 6
Effective Time: 20241001
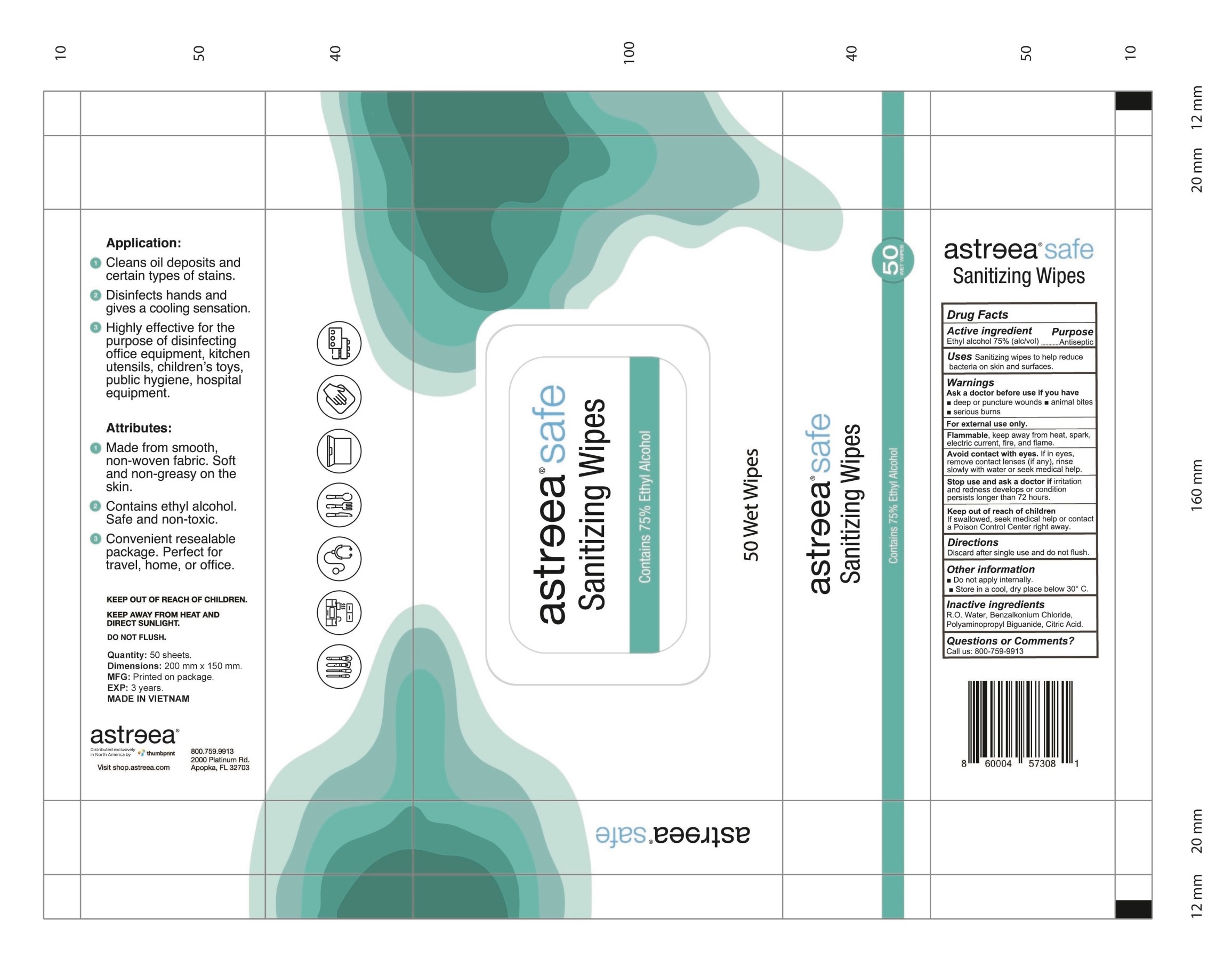 50 wipes NDC:
50 wipes NDC: