PLASMA-LYTE A- sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride injection, solution
Plasma-Lyte A by
Drug Labeling and Warnings
Plasma-Lyte A by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Company, Baxter Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Health Care Provider Letter
-
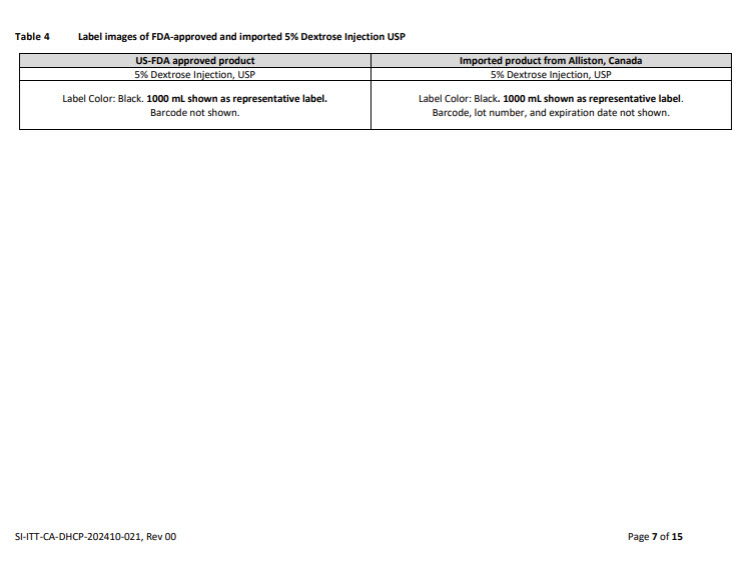
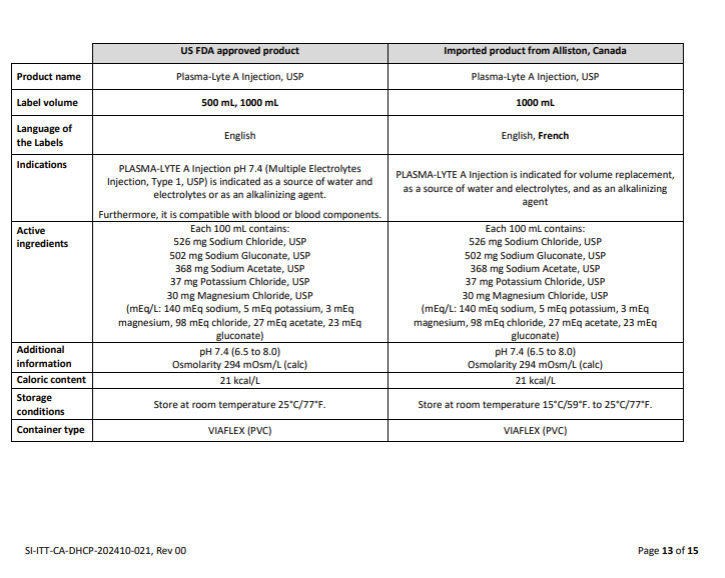
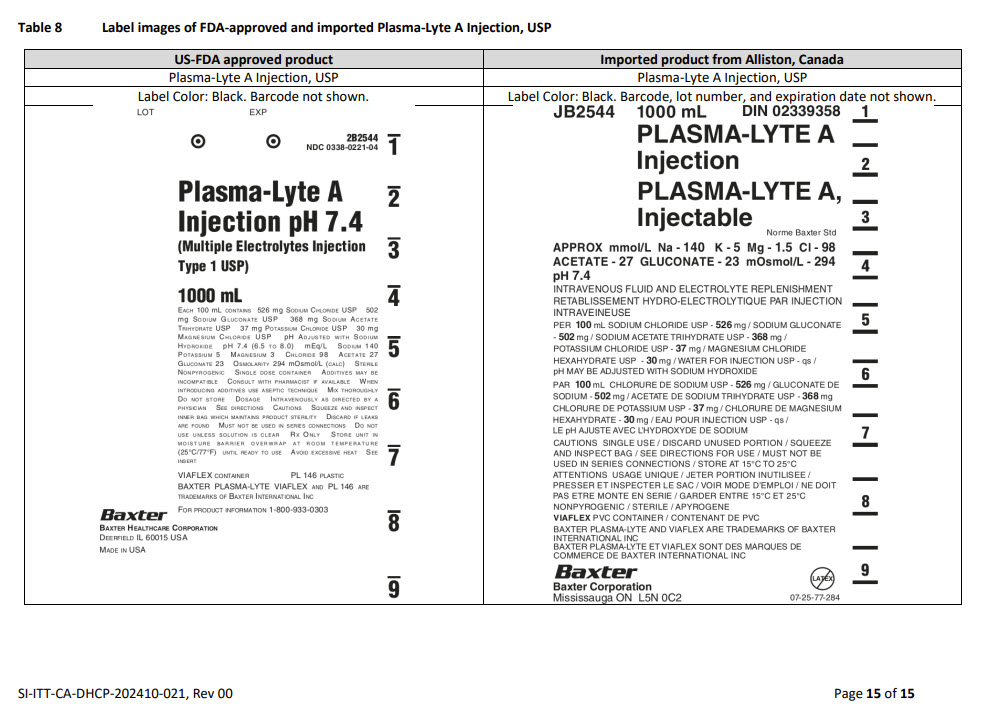
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
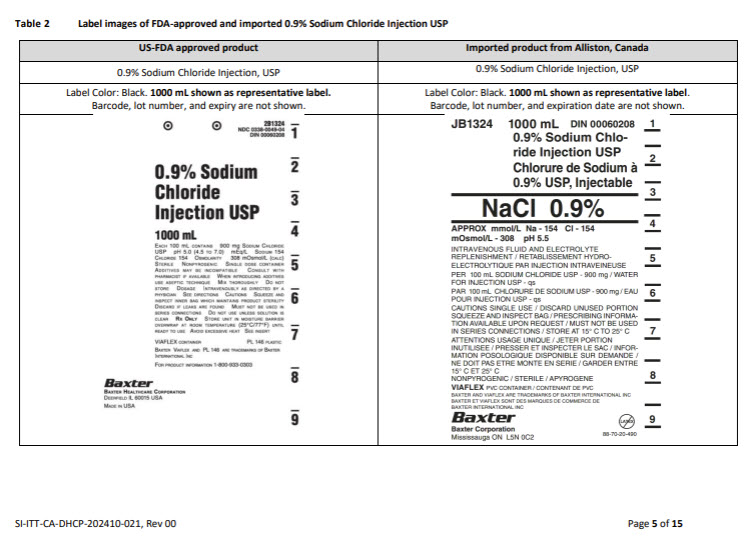
JB2544 1000 mL DIN 02339358
PLASMA-LYTE A
INJECTIONPLASMA-LYTE A,
InjectableNorme Baxter Std
APPROX mmol/L Na – 140 K – 5 Mg – 1.5 Cl – 98
ACETATE – 27 GLUCONATE – 23 mOsmol/L – 294
pH 7.4INTRAVENOUS FLUID AND ELECTROLYTE REPLENISHMENT
RETABLISSEMENT HYDRO-ELECTROLYTIQUE PAR INJECTION
INTRAVEINEUSEPER 100 mL SODIUM CHLORIDE USP – 526 mg / SODIUM GLUCONATE
- 502 mg / SODIUM ACETATE TRIHYDRATE USP – 368 mg /
POTASSIUM CHLORIDE USP – 37 mg / MAGNESIUM CHLORIDE
HEXAHYDRATE USP – 30 mg / WATER FOR INJECTION USP – qs
pH MAY BE ADJUSTED WITH SODIUM HYDROXIDEPAR 100 mL CHLORURE DE SODIUM USP – 526 mg / GLUCONATE DE
SODIUM - 502 mg / ACETATE DE SODIUM TRIHYDRATE USP – 368 mg
CHLORURE DE POTASSIUM USP – 37 mg / CHLORURE DE MAGNESIUM
HEXAHYDRATE USP – 30 mg / EAU POUR INJECTION USP – qs
LE pH AJUSTE AVEC L’HYDROXYDE DE SODIUMCAUTIONS SINGLE USE / DISCARD UNUSED PORTION / SQUEEZE
AND INSPECT BAG / SEE DIRECTIONS FOR USE / MUST NOT BE
USED IN SERIES CONNECTIONS / STORE AT 15°C TO 25°CATTENTIONS USAGE UNIQUE / JETER PORTION INUTILISEE/
PRESSER ET INSPECTER LE SAC / VOIR MODE D’EMPLOI / NE DOIT
PAS ETRE MONTE EN SERIE / GARDER ENTRE ET 15°C ET 25°CNONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER / CONTENANT DE PVC
BAXTER PLASMA-LYTE AND VIAFLEX ARE TRADEMARKS OF BAXTER
INTERNATIONAL INC
BAXTER PLASMA-LYTE ET VIAFLEX SONT DES MARQUES DE
COMMERCE DE BAXTER INTERNATIONAL INCBaxter Logo
Baxter Corporation
Mississauga ON L5C 0C2No LATEX Label
07-25-77-284
1
_
2
_
3
_
4
_
5
_
6
_
7
_
8
_
9
-
INGREDIENTS AND APPEARANCE
PLASMA-LYTE A
sodium chloride, sodium gluconate, sodium acetate, potassium chloride and magnesium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9591 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 526 mg in 100 mL SODIUM GLUCONATE (UNII: R6Q3791S76) (GLUCONIC ACID - UNII:R4R8J0Q44B, SODIUM CATION - UNII:LYR4M0NH37) SODIUM GLUCONATE 502 ug in 100 mL SODIUM ACETATE (UNII: 4550K0SC9B) (ACETATE ION - UNII:569DQM74SC, SODIUM CATION - UNII:LYR4M0NH37) SODIUM ACETATE 368 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 37 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 30 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9591-12 12 in 1 CARTON 11/06/2024 1 NDC: 0338-9591-01 1000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/06/2024 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Corporation 205087968 ANALYSIS(0338-9591) , LABEL(0338-9591) , MANUFACTURE(0338-9591) , STERILIZE(0338-9591) , PACK(0338-9591)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.