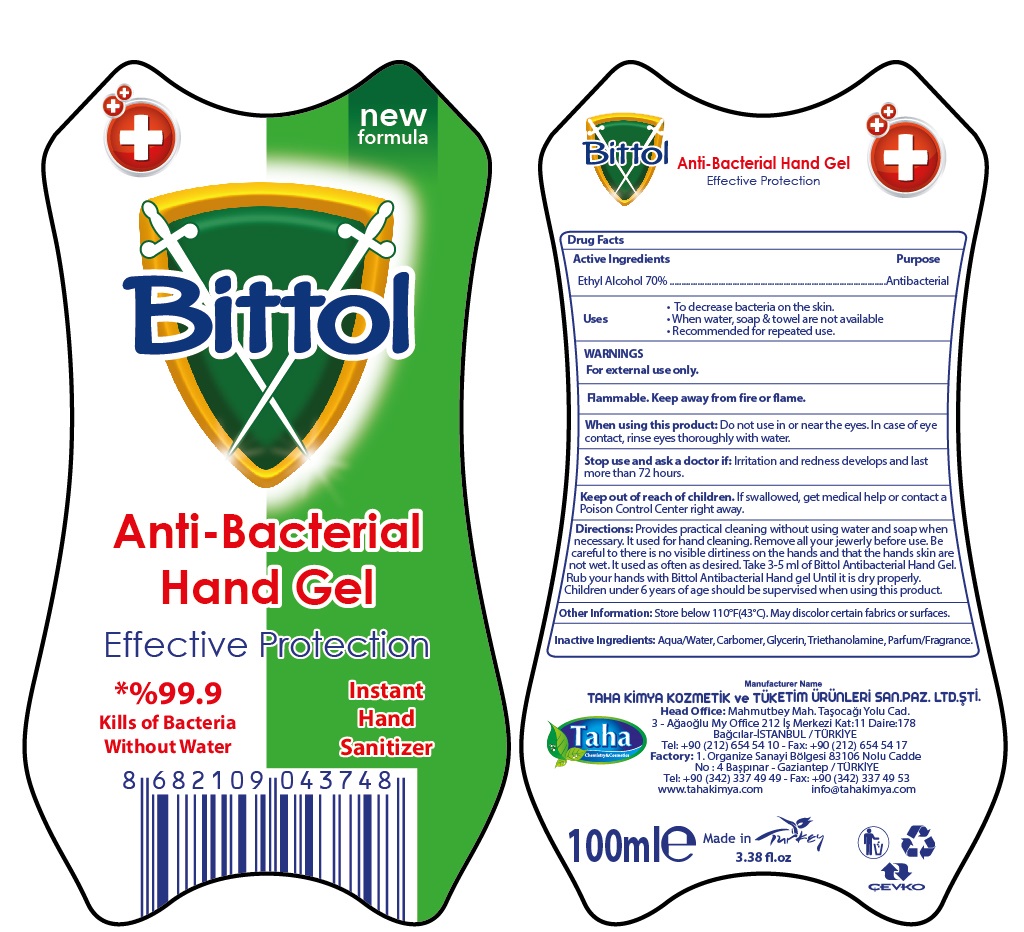
Bittol Anti-Bacterial Hand Gel
Bittol Anti-Bacterial Hand Gel by
Drug Labeling and Warnings
Bittol Anti-Bacterial Hand Gel by is a Otc medication manufactured, distributed, or labeled by Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BITTOL ANTI-BACTERIAL HAND GEL- alcohol gel
Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi
----------
Bittol Anti-Bacterial Hand Gel
Uses
- To decrease bacteria on the skin.
- When water, soap & towel are not available
- Recommended for repeated use.
Warnings
For external use only.
Flammable. Keep away from fire or flame.
Directions:
Provides practical cleaning without using water and soap when necessary. It used for hand cleaning. Remove all your jewerly before use. Be careful to there is no visible dirtiness on the hands and that the hands skin are not wet. It used as often as desired. Take 3-5 ml of Bittol Antibacterial Hand Gel. Rub your hands with Bittol Antibacterial Hand gel Until it is dry properly. Children under 6 years of age should be supervised when using this product.
| BITTOL ANTI-BACTERIAL HAND GEL
alcohol gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi (565657236) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.