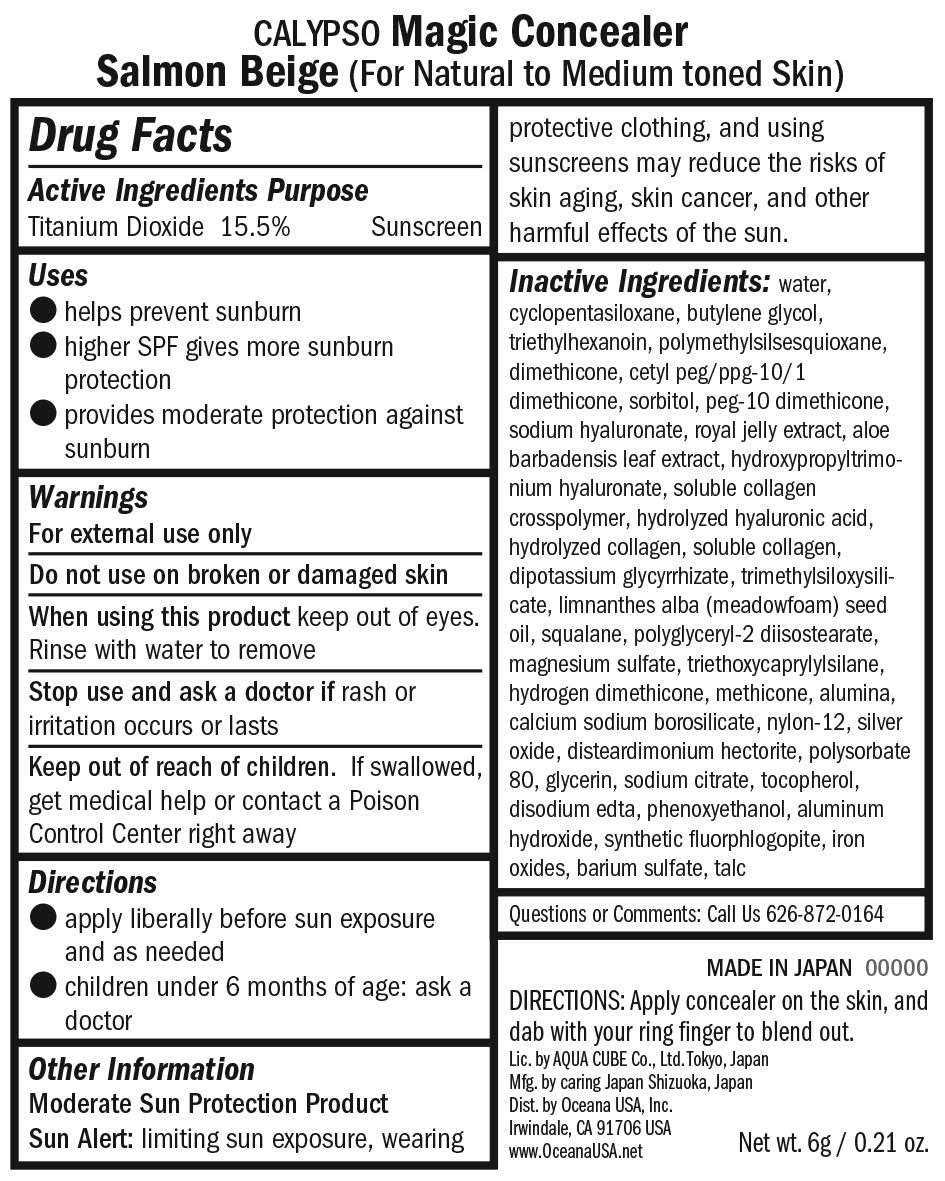
Calypso Magic Concealer Salmon Beige
Calypso Magic Concealer Salmon Beige by
Drug Labeling and Warnings
Calypso Magic Concealer Salmon Beige by is a Otc medication manufactured, distributed, or labeled by Aquacube Inc., Caring Japan Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CALYPSO MAGIC CONCEALER SALMON BEIGE- titanium dioxide lotion
Aquacube Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Calypso Magic Concealer Salmon Beige
Warnings
Warnings When using this product
- keep out of the eye area
- rinse with water to remove
- stop use and ask a doctor if rash or irritation develops
- for external use onlyl
- keep out of reach of children
Directions
- Apply liberally before sun exposure and as needed
- children under 6 months of age: ask a doctor
Inactive Ingredients
Inactive Ingredients:
water, cyclopentasiloxane, butylene glycol, triethylhexanoin, polymethylsilsesquioxane, dimethicone, cetyl PEG/PPG-10/1 dimethicone, sorbitol, PEG-10 dimethicone, sodium hyaluronate, royal jelly extract, aloe barbadensis leaf extract, hydroxypropyltrimonium hyaluronate, soluble collagen crosspolymer, hydrolyzed hyaluronic acid, hydrolyzed collagen, soluble collagen, dipotassium glycyrrhizate, trimethylsiloxysilicate, Limnanthes alba (meadowfoam) seed oil, squalene, polyglyceryl-2 diisostearate, magnesium sulfate, triethosycaprylylsilane, hydrogen dimethicone, methicone, alumina, calcium sodium borosilicate, nylon-12, silver oxide, disteardimonium hectorite, polysorbate 80, glycerin, sodium citrate, tocopherol, disodium EDTA, phenoxyethanol, aluminum hydroxide, synthetic fluorphlogopite, iron oxides, barium sulfate, talc
| CALYPSO MAGIC CONCEALER SALMON BEIGE
titanium dioxide lotion |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Aquacube Inc. (692311844) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Caring Japan Inc. | 702171786 | manufacture(79623-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.