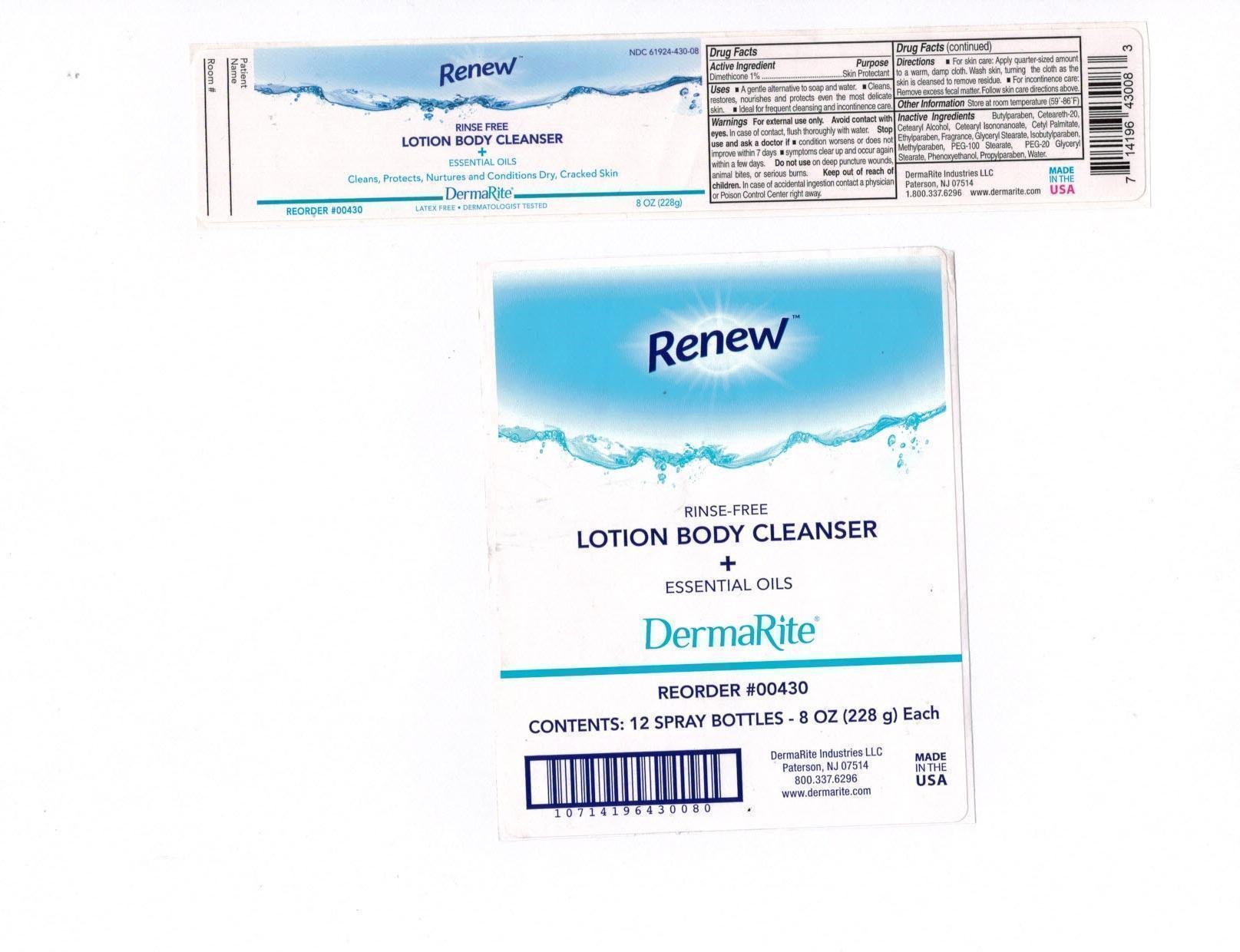
DRUG LISTING: RENEW BODY CLEANSER
RENEW BODY CLEANSER by
Drug Labeling and Warnings
RENEW BODY CLEANSER by is a Otc medication manufactured, distributed, or labeled by DermaRite Industries, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RENEW BODY CLEANSER- otc skin protectant drug products lotion
DermaRite Industries, LLC
----------
DRUG LISTING: RENEW BODY CLEANSER
Uses:
- A gentle alternative to soap and water.
- Cleans, restores, nourishes, and protects even most delicate skin.
- Ideal for frequent cleansing and incontinence care.
Warnings"
- For external use only.Avoid contact with eyes. In case of contact, flush thoroughly with water.
- Stop use and ask a doctor if – condition worsens or does not improve within 7 days; symptoms clear up and occur again within a few days.
- Do not use on deep puncture wounds, animal bites, or serious burns.
Warnings:
- Keep out of reach of children. In case of accidental ingestion contact a physician or Poison Control Center right away.
Directions:
- For skin care: Apply quarter-sized amount to warm, damp cloth. Wash skin, turning the cloth as the skin is cleansed to remove residue.
- For incontinence care: Remove excess fecal matter. Follow skin care directions above
| RENEW BODY CLEANSER
otc skin protectant drug products lotion |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - DermaRite Industries, LLC (883925562) |
| Registrant - DermaRite Industries, LLC (883925562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DermaRite Industries, LLC | 883925562 | manufacture(61924-430) | |
Revised: 12/2024
Document Id: 28880b59-b007-e343-e063-6394a90a1844
Set id: aa6dff13-b952-49ce-bd55-c6f58b303922
Version: 8
Effective Time: 20241205